(−)-nabilone
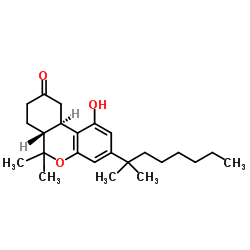
(−)-nabilone structure
|
Common Name | (−)-nabilone | ||
|---|---|---|---|---|
| CAS Number | 51022-71-0 | Molecular Weight | 372.541 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 457.4±45.0 °C at 760 mmHg | |
| Molecular Formula | C24H36O3 | Melting Point | 155-156ºC | |
| MSDS | USA | Flash Point | 145.4±22.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | (6aR,10aR)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan-2-yl)-7,8,10,10a-tetrahydro-6aH-benzo[c]chromen-9-one |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 457.4±45.0 °C at 760 mmHg |
| Melting Point | 155-156ºC |
| Molecular Formula | C24H36O3 |
| Molecular Weight | 372.541 |
| Flash Point | 145.4±22.2 °C |
| Exact Mass | 372.266449 |
| PSA | 46.53000 |
| LogP | 7.05 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.517 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ~18 mg/mL, soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 36/37/39-45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | HP8756000 |
|
Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain.
Expert Opin. Investig. Drugs 17(1) , 85-95, (2008) Nabilone has been approved to treat chemotherapy-induced nausea and vomiting. Recent studies have explored cannabinoids in pain management.To review the evidence for the use of cannabinoids in general... |
|
|
A randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury.
Arch. Phys. Med. Rehabil. 91(5) , 703-7, (2010) To determine whether nabilone, a synthetic cannabinoid, alleviates spasticity in people with spinal cord injury (SCI).A double-blind, placebo-controlled crossover study.Outpatient rehabilitation clini... |
|
|
An open-label comparison of nabilone and gabapentin as adjuvant therapy or monotherapy in the management of neuropathic pain in patients with peripheral neuropathy.
Pain Pract. 11(4) , 353-68, (2011) Neuropathic pain (NeP) is prevalent in patients with peripheral neuropathy (PN), regardless of etiology. We sought to compare the efficacy of the cannabinoid nabilone as either monotherapy or adjuvant... |
| 9H-Dibenzo(b,d)pyran-9-one, 3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-, (6aR,10aR)-rel- |
| UNII:2N4O9L084N |
| trans-(±)-3-(1,1-Dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-9H-dibenzo[b,d]pyran-9-one |
| Compound 109514 |
| nabilone |
| Nabilonum |
| (6aR,10aR)-1-Hydroxy-6,6-dimethyl-3-(2-methyloctan-2-yl)-6,6a,7,8,10,10a-hexahydro-9H-benzo[c]chromen-9-one |
| 9H-Dibenzo[b,d]pyran-9-one, 3-(1,1-dimethylheptyl)-6,6a,7,8,10,10a-hexahydro-1-hydroxy-6,6-dimethyl-, (6aR,10aR)- |
| (6aR,10aR)-nabilone |
| Cesamet |
| Nabilon |
| Nabilone (USAN/INN) |
| (6aR,10aR)-3-(1,1-Dimethylheptyl)-1-hydroxy-6,6-dimethyl-6,6a,7,8,10,10a-hexahydro-9H-benzo[c]chromen-9-on |
| (6aR,10aR)-3-(1,1-diméthylheptyl)-1-hydroxy-6,6-diméthyl-6,6a,7,8,10,10a-hexahydro-9H-benzo[c]chromén-9-one |
| Cesamet (TN) |
| (6aR,10aR)-1-Hydroxy-6,6-dimethyl-3-(2-methyl-2-octanyl)-6,6a,7,8,10,10a-hexahydro-9H-benzo[c]chromen-9-one |
| Nabilona |
| (6aR,10aR)-3-(1,1-dimethylheptyl)-1-hydroxy-6,6-dimethyl-6,6a,7,8,10,10a-hexahydro-9H-benzo[c]chromen-9-one |