| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L-Phenylalanine
CAS:63-91-2 |
|
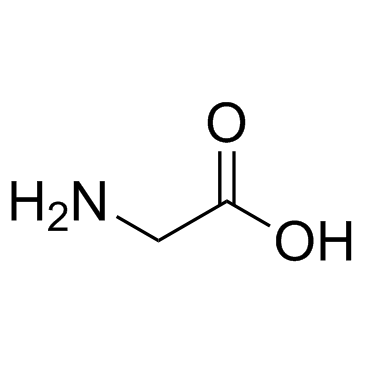 |
Glycine
CAS:56-40-6 |
|
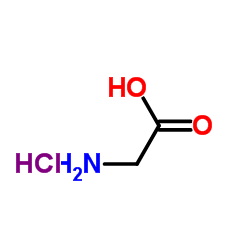 |
Glycine HCl
CAS:6000-43-7 |