L-Phenylalanine

L-Phenylalanine structure
|
Common Name | L-Phenylalanine | ||
|---|---|---|---|---|
| CAS Number | 63-91-2 | Molecular Weight | 165.189 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 307.5±30.0 °C at 760 mmHg | |
| Molecular Formula | C9H11NO2 | Melting Point | 270-275ºC (dec.)(lit.) | |
| MSDS | USA | Flash Point | 139.8±24.6 °C | |
Use of L-PhenylalanineL-Phenylalanine is an antagonist at α2δ calcium channels with a Ki of 980 nM. IC50 Value: 980 nM [1]Target: Calcium ChannelL-Phenylalanine (LPA) is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form proteins. In the brain, L-phenylalanine is a competitive antagonist at the glycine binding site of NMDA receptor and at the glutamate binding site of AMPA receptor [2, 3]. At the glycine binding site of NMDA receptor L-phenylalanine has an apparent equilibrium dissociation constant (KB) of 573 ?M estimated by Schild regression [4] which is considerably lower than brain L-phenylalanine concentration observed in untreated human phenylketonuria [5]. L-Phenylalanine also inhibits neurotransmitter release at glutamatergic synapses in hippocampus and cortex with IC50 of 980 nM, a brain concentration seen in classical phenylketonuria, whereas D-phenylalanine has a significantly smaller effect [3]. |
| Name | L-phenylalanine |
|---|---|
| Synonym | More Synonyms |
| Description | L-Phenylalanine is an antagonist at α2δ calcium channels with a Ki of 980 nM. IC50 Value: 980 nM [1]Target: Calcium ChannelL-Phenylalanine (LPA) is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form proteins. In the brain, L-phenylalanine is a competitive antagonist at the glycine binding site of NMDA receptor and at the glutamate binding site of AMPA receptor [2, 3]. At the glycine binding site of NMDA receptor L-phenylalanine has an apparent equilibrium dissociation constant (KB) of 573 ?M estimated by Schild regression [4] which is considerably lower than brain L-phenylalanine concentration observed in untreated human phenylketonuria [5]. L-Phenylalanine also inhibits neurotransmitter release at glutamatergic synapses in hippocampus and cortex with IC50 of 980 nM, a brain concentration seen in classical phenylketonuria, whereas D-phenylalanine has a significantly smaller effect [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 307.5±30.0 °C at 760 mmHg |
| Melting Point | 270-275ºC (dec.)(lit.) |
| Molecular Formula | C9H11NO2 |
| Molecular Weight | 165.189 |
| Flash Point | 139.8±24.6 °C |
| Exact Mass | 165.078979 |
| PSA | 63.32000 |
| LogP | 1.11 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.576 |
| InChIKey | COLNVLDHVKWLRT-QMMMGPOBSA-N |
| SMILES | NC(Cc1ccccc1)C(=O)O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | C: Corrosive; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 22-24/25-37/39-45-36/37/39-27-26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | AY7535000 |
| HS Code | 29224995 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 29224995 |
|---|
|
Assessment of protein modifications in liver of rats under chronic treatment with paracetamol (acetaminophen) using two complementary mass spectrometry-based metabolomic approaches.
J. Proteomics 120 , 194-203, (2015) Liver protein can be altered under paracetamol (APAP) treatment. APAP-protein adducts and other protein modifications (oxidation/nitration, expression) play a role in hepatotoxicity induced by acute o... |
|
|
Enhanced Indirect Photochemical Transformation of Histidine and Histamine through Association with Chromophoric Dissolved Organic Matter.
Environ. Sci. Technol. 49 , 5511-9, (2015) Photochemical transformations greatly affect the stability and fate of amino acids (AAs) in sunlit aquatic ecosystems. Whereas the direct phototransformation of dissolved AAs is well investigated, the... |
|
|
Use of Commercial Dry Yeast Products Rich in Mannoproteins for White and Rosé Sparkling Wine Elaboration.
J. Agric. Food Chem. 63 , 5670-81, (2015) In sparkling wines, mannoproteins released during yeast autolysis largely affect their final quality. This process is very slow and may take several months. The aim of this work was to study the effec... |
| Benzenepropanethioic acid,S-phenyl ester |
| (S)-phenylalanine |
| L-Phenylalanine |
| EINECS 200-568-1 |
| (2S)-2-amino-3-phenylpropanoic acid |
| L-PHENYLALININE |
| H-Phe-OH |
| L-phenylalanine zwitterion |
| S-phenylalanine |
| (S)-(-)-Phenylalanine |
| Thiohydrozimtsaeurephenylester |
| (S)-a-Amino-b-phenylpropionic Acid |
| L-2-Amino-3-phenylpropionic acid |
| (S)-2-Amino-3-phenylpropionic acid |
| (S)-2-Amino-3-phenylpropanoic acid |
| C6H5CH2CH(NH2)COOH |
| (S)-a-Aminohydrocinnamic Acid |
| Phenylalanine |
| (L)-Phenylalanine |
| Phenylalanine (VAN) |
| l-Phe |
| PhE |
| phenyl 4-phenylthiobutanoate |
| S-Phenyl-3-phenylpropanthioat |
| β-phenyl-L-alanine |
| (-)-Phenylalanine |
| (S)-α-Aminobenzenepropanoic acid |
| MFCD00064227 |
| 3-Phenylpropionyl-phenyl-thioether |
| α-Amino-β-phenylpropionic acid |
| 3-Phenylthiopropionic acid,S-phenyl ester |
| (S)-A-Aminobenzenepropanoic Acid |
| Acetylcysteine Impurity 3 |
![3-phenyl-(2S)-[(1'R)-phenylethylamino]propionic acid Structure](https://image.chemsrc.com/caspic/203/1004755-04-7.png) CAS#:1004755-04-7
CAS#:1004755-04-7 CAS#:1202-31-9
CAS#:1202-31-9 CAS#:150-30-1
CAS#:150-30-1 CAS#:55895-88-0
CAS#:55895-88-0 CAS#:100-39-0
CAS#:100-39-0 CAS#:962-39-0
CAS#:962-39-0 CAS#:33305-77-0
CAS#:33305-77-0![Benzenepropanoic acid,-[(1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl)oxy]-,(aR) structure](https://image.chemsrc.com/caspic/117/310404-47-8.png) CAS#:310404-47-8
CAS#:310404-47-8 CAS#:7400-08-0
CAS#:7400-08-0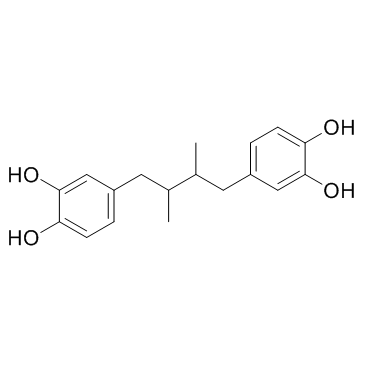 CAS#:500-38-9
CAS#:500-38-9 CAS#:57060-88-5
CAS#:57060-88-5 CAS#:37553-65-4
CAS#:37553-65-4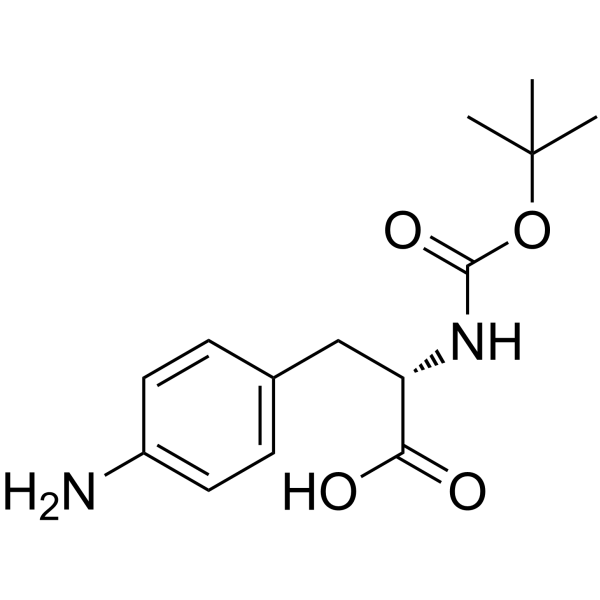 CAS#:55533-24-9
CAS#:55533-24-9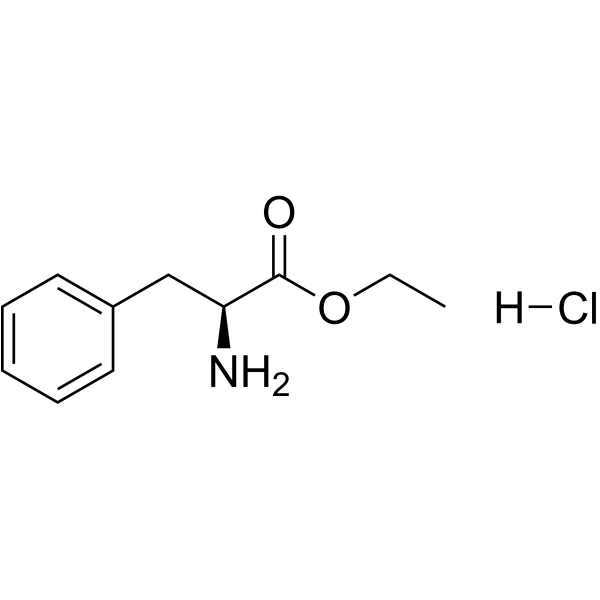 CAS#:3182-93-2
CAS#:3182-93-2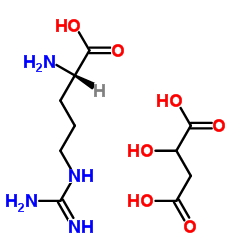 CAS#:41994-51-8
CAS#:41994-51-8 CAS#:37736-82-6
CAS#:37736-82-6
