| Structure | Name/CAS No. | Articles |
|---|---|---|
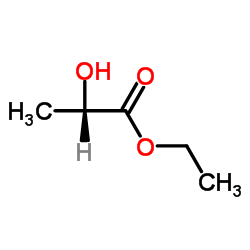 |
(−)-Ethyl L-lactate
CAS:687-47-8 |
|
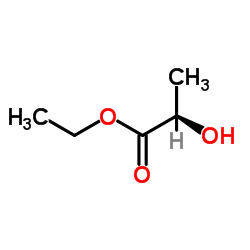 |
(R)-ethyl 2-hydroxypropanoate
CAS:7699-00-5 |
|
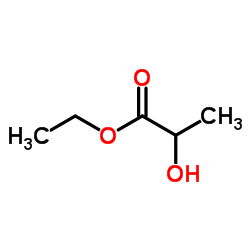 |
Ethyl lactate
CAS:97-64-3 |