Purification and properties of a carbonyl reductase involved in stereoselective reduction of ethyl 4-chloro-3-oxobutanoate from Cylindrocarpon sclerotigenum IFO 31855.
Yuri Saratani, Eiji Uheda, Hiroaki Yamamoto, Atsuo Nishimura, Fumiki Yoshizako
Index: Biosci. Biotechnol. Biochem. 67(6) , 1417-20, (2003)
Full Text: HTML
Abstract
A NADPH-dependent carbonyl reductase (CSCR1) was purified to homogeneity from Cylindrocarpon sclerotigenum IFO 31855. The enzyme catalyzed the stereoselective reduction of ethyl 4-chloro-3-oxobutanoate to the corresponding (S)-alcohol with a >99% enantiomer excess. The relative molecular mass of the enzyme was estimated to be 68,000 by gel filtration chromatography and 24,800 on SDS polyacrylamide gel electrophoresis. The enzyme had an extremely narrow substrate specificity and it highly reduced conjugated diketone, 2,3-butanedion, in addition to ethyl 4-chloro-3-oxobutanoate. The enzyme activity was inhibited by HgCl(2) (100%), 5,5'-dithiobis(2-nitrobenzoic acid) (56%), dicoumarol (42%), and CuSO(4) (46%). The N-terminal amino acid sequence of the enzyme (P-Q-G-I-P-T-A-S-R-L) showed no apparent similarity with those of other oxidoreductases.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
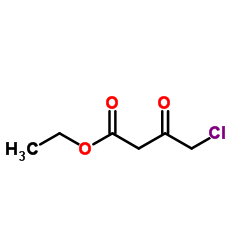 |
Ethyl 2-Chloroacetoacetate
CAS:638-07-3 |
C6H9ClO3 |
|
Construction of a two-strain system for asymmetric reduction...
2006-03-01 [Appl. Microbiol. Biotechnol. 70(1) , 40-6, (2006)] |
|
Purification and characterization of an alpha-haloketone-res...
2003-10-01 [Biosci. Biotechnol. Biochem. 67(10) , 2145-53, (2003)] |
|
A novel carbonyl reductase from Pichia stipitis for the prod...
2009-04-01 [Biotechnol. Lett. 31(4) , 537-42, (2009)] |
|
Biosimulation of drug metabolism—A yeast based model
2009-01-01 [Eur. J. Pharm. Sci. 36(1) , 157-70, (2009)] |
|
Enantioselective bioconversion using Escherichia coli cells ...
2010-09-01 [J. Microbiol. Biotechnol. 20(9) , 1300-6, (2010)] |