The population pharmacokinetics of R- and S-warfarin: effect of genetic and clinical factors.
Steven Lane, Sameh Al-Zubiedi, Ellen Hatch, Ivan Matthews, Andrea L Jorgensen, Panos Deloukas, Ann K Daly, B Kevin Park, Leon Aarons, Kayode Ogungbenro, Farhad Kamali, Dyfrig Hughes, Munir Pirmohamed
Index: Br. J. Clin. Pharmacol. 73(1) , 66-76, (2012)
Full Text: HTML
Abstract
Warfarin is a drug with a narrow therapeutic index and large interindividual variability in daily dosing requirements. Patients commencing warfarin treatment are at risk of bleeding due to excessive anticoagulation caused by overdosing. The interindividual variability in dose requirements is influenced by a number of factors, including polymorphisms in genes mediating warfarin pharmacology, co-medication, age, sex, body size and diet.To develop population pharmacokinetic models of both R- and S-warfarin using clinical and genetic factors and to identify the covariates which influence the interindividual variability in the pharmacokinetic parameters of clearance and volume of distribution in patients on long-term warfarin therapy.Patients commencing warfarin therapy were followed up for 26 weeks. Plasma warfarin enantiomer concentrations were determined in 306 patients for S-warfarin and in 309 patients for R-warfarin at 1, 8 and 26 weeks. Patients were also genotyped for CYP2C9 variants (CYP2C9*1,*2 and *3), two single-nucleotide polymorphisms (SNPs) in CYP1A2, one SNP in CYP3A4 and six SNPs in CYP2C19. A base pharmacokinetic model was developed using NONMEM software to determine the warfarin clearance and volume of distribution. The model was extended to include covariates that influenced the between-subject variability.Bodyweight, age, sex and CYP2C9 genotype significantly influenced S-warfarin clearance. The S-warfarin clearance was estimated to be 0.144 l h⁻¹ (95% confidence interval 0.131, 0.157) in a 70 kg woman aged 69.8 years with the wild-type CYP2C9 genotype, and the volume of distribution was 16.6 l (95% confidence interval 13.5, 19.7). Bodyweight and age, along with the SNPs rs3814637 (in CYP2C19) and rs2242480 (in CYP3A4), significantly influenced R-warfarin clearance. The R-warfarin clearance was estimated to be 0.125 l h⁻¹ (95% confidence interval 0.115, 0.135) in a 70 kg individual aged 69.8 years with the wild-type CYP2C19 and CYP3A4 genotypes, and the volume of distribution was 10.9 l (95% confidence interval 8.63, 13.2).Our analysis, based on exposure rather than dose, provides quantitative estimates of the clinical and genetic factors impacting on the clearance of both the S- and R-enantiomers of warfarin, which can be used in developing improved dosing algorithms.© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
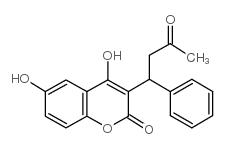 |
6-hydroxy Warfarin
CAS:17834-02-5 |
C19H16O5 |
|
Investigation of two-dimensional high performance liquid chr...
2014-10-10 [J. Chromatogr. A. 1363 , 200-6, (2014)] |
|
Hydroxywarfarin metabolites potently inhibit CYP2C9 metaboli...
2010-05-17 [Chem. Res. Toxicol. 23(5) , 939-45, (2010)] |
|
Metabolism of R- and S-warfarin by CYP2C19 into four hydroxy...
2012-09-01 [Drug Metab. Lett. 6(3) , 157-64, (2012)] |
|
Warfarin is an effective modifier of multiple UDP-glucuronos...
2015-01-01 [J. Pharm. Sci. 104(1) , 244-56, (2014)] |
|
Analysis of R- and S-hydroxywarfarin glucuronidation catalyz...
2012-01-01 [J. Pharmacol. Exp. Ther. 340(1) , 46-55, (2012)] |