| Structure | Name/CAS No. | Articles |
|---|---|---|
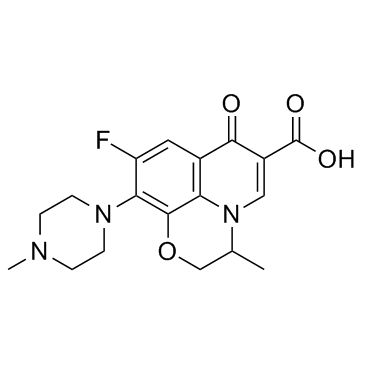 |
Ofloxacin
CAS:82419-36-1 |
|
 |
Nitrendipine
CAS:39562-70-4 |
|
 |
4-AMINOPYRIDINE
CAS:504-24-5 |
|
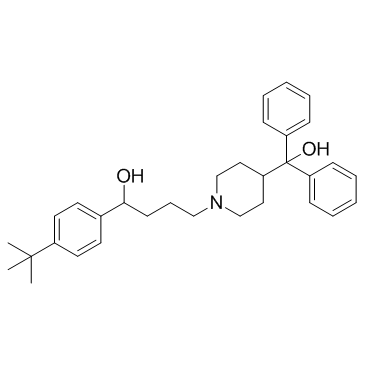 |
Terfenadine
CAS:50679-08-8 |
|
 |
Caffeine
CAS:58-08-2 |
|
 |
Trimethoprim
CAS:738-70-5 |
|
 |
Clotrimazole
CAS:23593-75-1 |
|
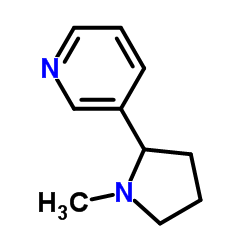 |
(±)-nicotine
CAS:22083-74-5 |
|
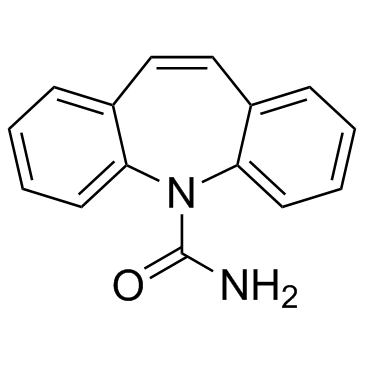 |
Carbamazepine
CAS:298-46-4 |
|
 |
Tamoxifen
CAS:10540-29-1 |