Treatment of infantile spasms with sodium valproate followed by benzodiazepines.
Narong Auvichayapat, Sompon Tassniyom, Sutthinee Treerotphon, Paradee Auvichayapat
Index: J. Med. Assoc. Thai. 90(9) , 1809-14, (2007)
Full Text: HTML
Abstract
To review the result of the infantile spasms' treatment with sodium valproate followed by nitrazepam or clonazepam.Descriptive retrospective study.Srinagarind Hospital, Department of Pediatrics, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand.Twenty-four infantile spasms admitted between January 1994 and December 2003 were analyzed. The inclusion criteria were the patients with infantile spasms clinically diagnosed by the pediatric neurologist, having hypsarrhythmic pattern EEG, and receiving sodium valproate with or without nitrazepam or clonazepam. The patients who had an uncertain diagnosis, incomplete medical record, or that were incompletely followed up were excluded. Data were collected on sex, age at onset of seizure, type of infantile spasms, associated type of seizure, predisposing etiological factor, neuroimaging study, and the result of treatment including cessation of spasms, subsequent development of other seizure types, quantitative reduction of spasms, relapse rates of spasms, psychomotor development, and adverse effects of AEDs.The mean age at onset was 177 days. The male-to-female ratio was 1:1.2. There were 13 cryptogenic (54.2%) and 11 symptomatic (45.8%) infantile spasms. The most common predisposing etiological factors in symptomatic cases were hypoxic ischemic encephalopathy (45.5%) and microcephaly (36.4%), respectively. Ten patients received sodium valproate (41.7%), another 10 received sodium valproate with clonazepam (41.7%), and four received sodium valproate with nitrazepam (16.7%). Both, the complete cessation rate and the 50% reduction of spasms rate were 45.8%. The duration to complete cessation was 70 days. The relapse rate was 18.2%. The rate of delayed psychomotor development was 83.3%. The mean duration of follow-up was 49.6 months.The authors propose to use sodium valproate concomitantly with benzodiazepines, especially clonazepam, in situations such as unavailability, intolerability, or adverse effects of ACTH or vigabatrin, or in a patient who does not respond to ACTH or vigabatrin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
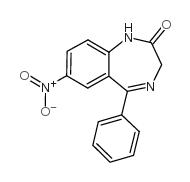 |
Nitrazepam
CAS:146-22-5 |
C15H11N3O3 |
|
Sedative-hypnotics are widely abused by drivers apprehended ...
2015-06-01 [Ther. Drug Monit. 37 , 339-46, (2015)] |
|
Determination of low levels of benzodiazepines and their met...
2015-01-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 975 , 24-33, (2014)] |
|
Cleaning up blood samples using a modified "QuEChERS" proced...
2014-10-01 [Forensic Sci. Int. 243 , 99-106, (2014)] |
|
Simple and sensitive screening and quantitative determinatio...
2014-11-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 970 , 1-7, (2014)] |
|
Urine Mescaline Screening With a Biochip Array Immunoassay a...
2015-12-01 [Ther. Drug Monit. 37 , 805-11, (2015)] |