| Structure | Name/CAS No. | Articles |
|---|---|---|
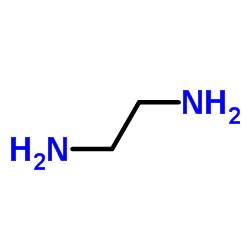 |
1,2-Ethanediamine
CAS:107-15-3 |
|
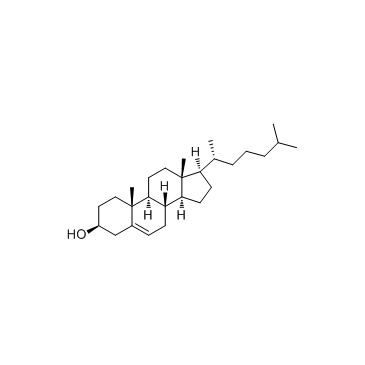 |
cholesterol
CAS:57-88-5 |
|
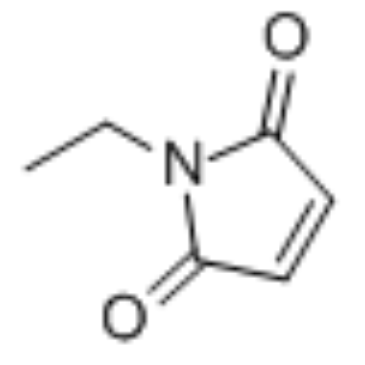 |
N-ethylmaleimide
CAS:128-53-0 |
|
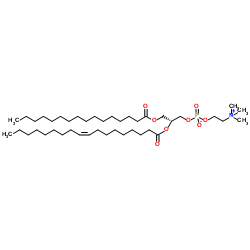 |
1-Palmitoyl-2-oleoyl-sn-glycero-3-PC
CAS:26853-31-6 |
|
 |
5(6)-Carboxyfluorescein
CAS:72088-94-9 |
|
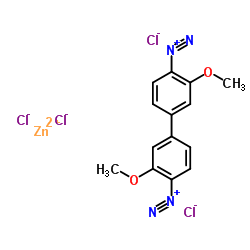 |
Fast Blue B Salt
CAS:14263-94-6 |