| Structure | Name/CAS No. | Articles |
|---|---|---|
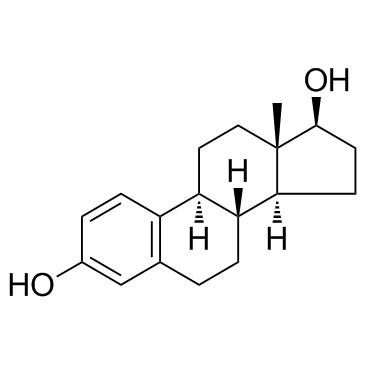 |
beta-Estradiol
CAS:50-28-2 |
|
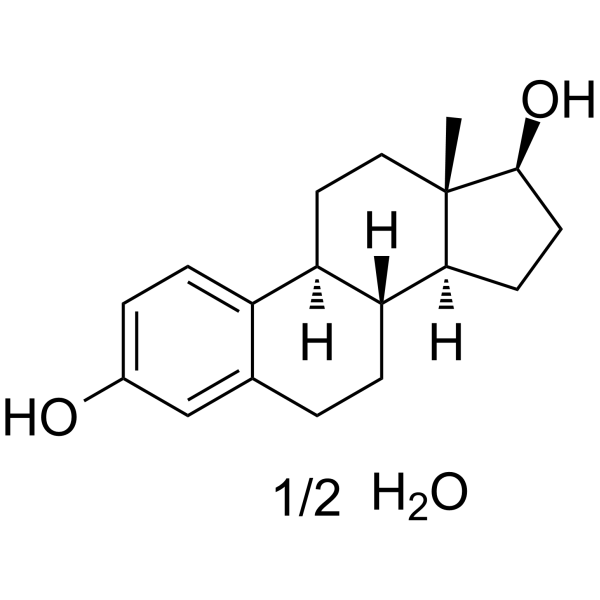 |
ESTRADIOL HEMIHYDRATE
CAS:35380-71-3 |
|
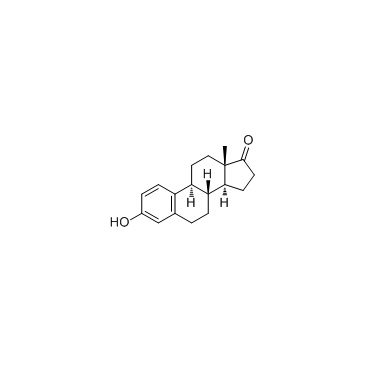 |
Estrone
CAS:53-16-7 |
|
 |
6-Ketoestradiol
CAS:571-92-6 |