| Structure | Name/CAS No. | Articles |
|---|---|---|
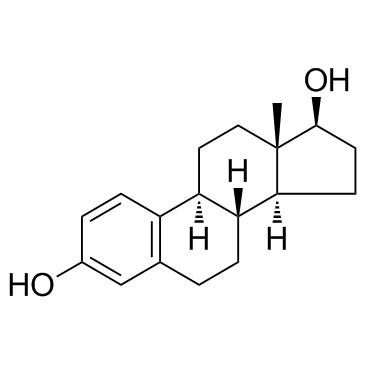 |
beta-Estradiol
CAS:50-28-2 |
|
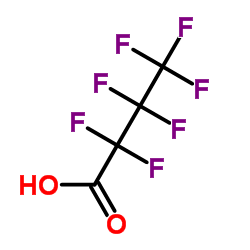 |
Heptafluorobutyric acid
CAS:375-22-4 |
|
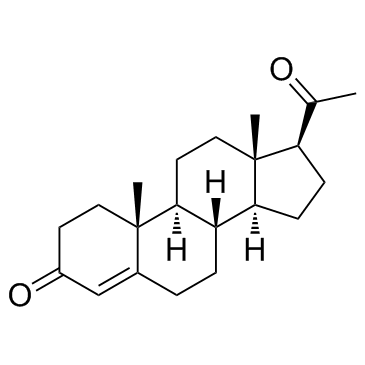 |
Progesterone
CAS:57-83-0 |
|
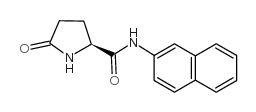 |
L-pyroglutamic acid beta-naphthylamide
CAS:22155-91-5 |
|
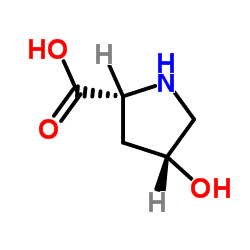 |
cis-4-Hydroxyproline
CAS:2584-71-6 |
|
 |
L-Hydroxyproline
CAS:51-35-4 |