Partitioning and confinement of GM1 ganglioside induced by amyloid aggregates.
Martino Calamai, Francesco S Pavone
Index: FEBS Lett. 587(9) , 1385-91, (2013)
Full Text: HTML
Abstract
Growing evidence shows that GM1 ganglioside is involved in amyloid deposition and toxicity. By means of real-time single particle tracking, we show that amyloid oligomers and aggregates formed by Aβ1-42 and amylin, two peptides associated, respectively, with the development of Alzheimer's disease and type II diabetes, interact with GM1 and decrease dramatically its lateral diffusion on the plasma membrane of living neuroblastoma cells. The confinement of GM1, a constituent of membrane rafts involved in neuroprotection, at the level of both types of amyloid aggregates can interfere with cell signaling pathways and contribute to the loss of neuroprotection.Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
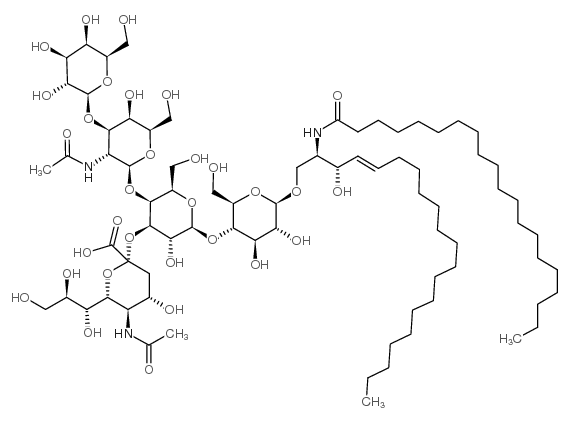 |
Ganglioside GM1
CAS:37758-47-7 |
C77H139N3O31 |
|
Methylmercury causes neuronal cell death through the suppres...
2015-02-01 [Toxicol. Appl. Pharmacol. 282(3) , 259-66, (2015)] |
|
Ganglioside GM1-mediated transcytosis of cholera toxin bypas...
2013-09-06 [J. Biol. Chem. 288(36) , 25804-9, (2013)] |
|
Fuzheng Huayu inhibits carbon tetrachloride-induced liver fi...
2013-01-09 [J. Ethnopharmacol. 145(1) , 175-81, (2013)] |
|
Molecular evolution of peptide ligands with custom-tailored ...
2012-01-01 [PLoS Comput. Biol. 8(12) , e1002800, (2012)] |
|
Altered levels of α-synuclein and sphingolipids in Batten di...
2014-04-15 [Gene 539(2) , 181-5, (2014)] |