| Structure | Name/CAS No. | Articles |
|---|---|---|
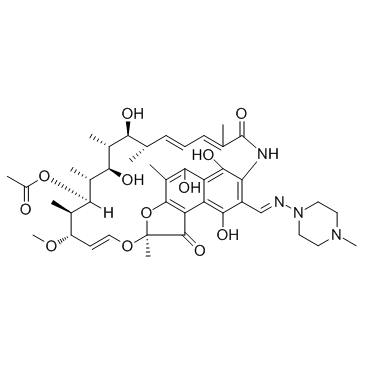 |
Rifampicin
CAS:13292-46-1 |
|
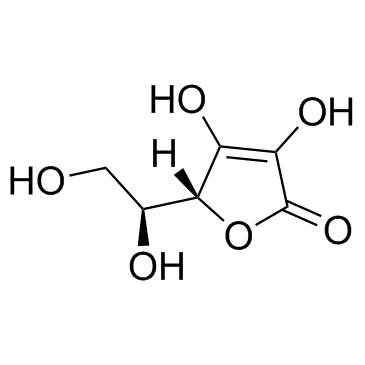 |
Ascorbic acid
CAS:50-81-7 |
|
 |
Ibuprofen
CAS:15687-27-1 |
|
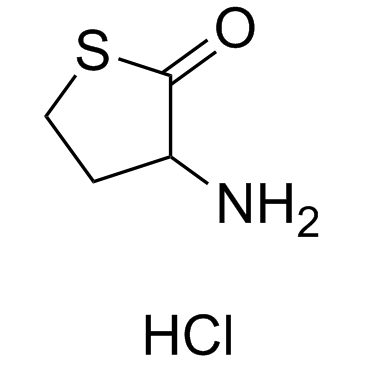 |
3-Aminodihydro-2(3H)-thiophenone hydrochloride
CAS:6038-19-3 |
|
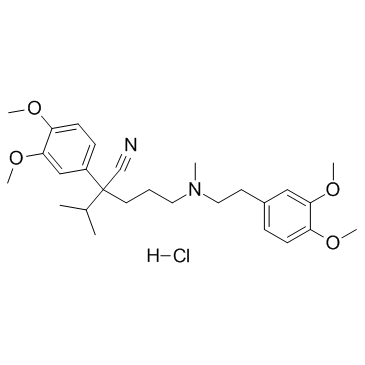 |
Verapamil HCl
CAS:152-11-4 |
|
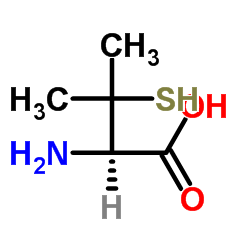 |
L-(+)-Penicillamine
CAS:52-66-4 |
|
 |
Warfarin
CAS:81-81-2 |
|
 |
D-penicillamine
CAS:52-67-5 |
|
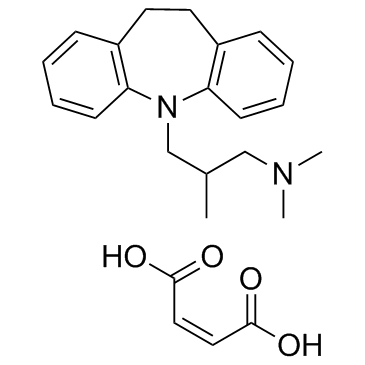 |
Trimipramine maleate
CAS:521-78-8 |