| Structure | Name/CAS No. | Articles |
|---|---|---|
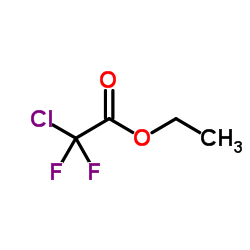 |
Ethyl chloro(difluoro)acetate
CAS:383-62-0 |
|
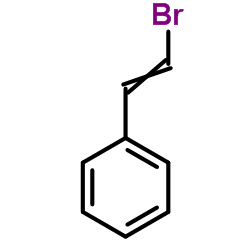 |
(2-Bromovinyl)benzene
CAS:103-64-0 |