| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Forskolin
CAS:66575-29-9 |
|
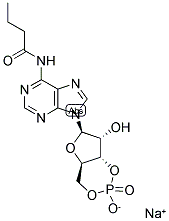 |
N6-Monobutyryladenosine 3′:5′-cyclic monophosphate sodium salt
CAS:70253-67-7 |