| Structure | Name/CAS No. | Articles |
|---|---|---|
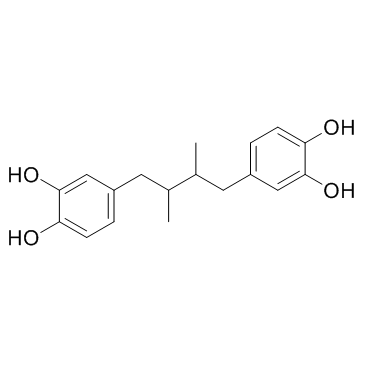 |
Nordihydroguaiaretic acid
CAS:500-38-9 |
|
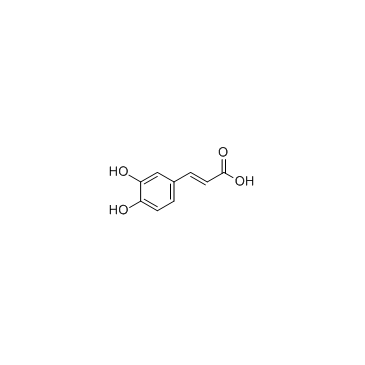 |
Caffeic acid
CAS:331-39-5 |
|
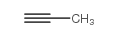 |
Propyne
CAS:74-99-7 |
|
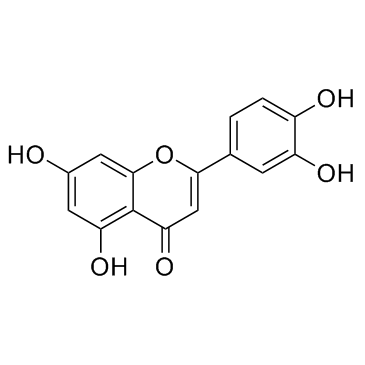 |
Luteolin
CAS:491-70-3 |