Acidic residue modifications restore chaperone activity of β-casein interacting with lysozyme.
A A Moosavi-Movahedi, H Rajabzadeh, M Amani, D Nourouzian, K Zare, H Hadi, A Sharifzadeh, N Poursasan, F Ahmad, N Sheibani
Index: Int. J. Biol. Macromol. 49(4) , 616-21, (2011)
Full Text: HTML
Abstract
An important factor in medicine and related industries is the use of chaperones to reduce protein aggregation. Here we show that chaperone ability is induced in β-casein by modification of its acidic residues using Woodward's Reagent K (WRK). Lysozyme at pH 7.2 was used as a target protein to study β-casein chaperone activities. The mechanism for chaperone activity of the modified β-casein was determined using UV-vis absorbencies, fluorescence spectroscopy, differential scanning calorimetry and theoretical calculations. Our results indicated that the β-casein destabilizes the lysozyme and increases its aggregation rate. However, WRK-ring sulfonate anion modifications enhanced the hydrophobicity of β-casein resulting in its altered net negative charge upon interactions with lysozyme. The reversible stability of lysozyme increased in the presence of WRK-modified β-casein, and hence its aggregation rate decreased. These results demonstrate the enhanced chaperone activity of modified β-casein and its protective effects on lysozyme refolding.Copyright © 2011 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
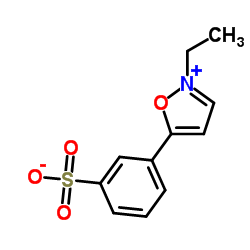 |
n-ethyl-5-phenylisoxazolium-3'-sulfonate
CAS:4156-16-5 |
C11H11NO4S |
|
[Synthesis of electroconductive polyaniline using immobilize...
2009-01-01 [Prikl. Biokhim. Mikrobiol. 45(1) , 33-7, (2009)] |
|
The carboxyl side chain of glutamate 681 interacts with a ch...
2003-02-18 [Biochemistry 42(6) , 1589-602, (2003)] |
|
Effect of protein-modifying reagents on ecto-apyrase from ra...
2000-01-01 [Int. J. Biochem. Cell Biol. 32(1) , 105-13, (2000)] |
|
Persistence of external chloride and DIDS binding after chem...
1999-10-01 [Am. J. Physiol. 277(4 Pt 1) , C791-9, (1999)] |
|
Woodward's reagent K inactivation of Escherichia coli L-thre...
1996-02-01 [Protein Sci. 5(2) , 382-90, (1996)] |