n-ethyl-5-phenylisoxazolium-3'-sulfonate
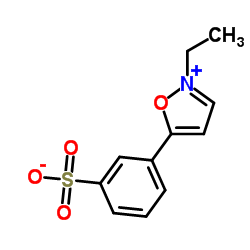
n-ethyl-5-phenylisoxazolium-3'-sulfonate structure
|
Common Name | n-ethyl-5-phenylisoxazolium-3'-sulfonate | ||
|---|---|---|---|---|
| CAS Number | 4156-16-5 | Molecular Weight | 253.274 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H11NO4S | Melting Point | 216-219 °C (dec.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
[Synthesis of electroconductive polyaniline using immobilized laccase].
Prikl. Biokhim. Mikrobiol. 45(1) , 33-7, (2009) A new method for synthesis of the conductive complex between polyaniline (PANI) and poly(2-acrylamido-2-methyl-1-propanosulfonic acid) (PAMPS) was proposed; in this method, the immobilized laccase from the basidiomycete Trametes hirsuta is used as a biocataly... |
|
|
The carboxyl side chain of glutamate 681 interacts with a chloride binding modifier site that allosterically modulates the dimeric conformational state of band 3 (AE1). Implications for the mechanism of anion/proton cotransport.
Biochemistry 42(6) , 1589-602, (2003) Glutamate 681 is thought to be located within the transport channel of band 3 (AE1, the chloride/bicarbonate exchanger), where it acts as a proton donor for the anion/proton cotransport function. Here we show that neutralization of the negative charge on glut... |
|
|
Effect of protein-modifying reagents on ecto-apyrase from rat brain.
Int. J. Biochem. Cell Biol. 32(1) , 105-13, (2000) We have tested several chemical modifiers to investigate which amino acid residues, present in the primary structure of the ecto-apyrase, could be involved in catalysis. Synaptosomes from cerebral cortex of rats were prepared and the ATP diphosphohydrolase ac... |
|
|
Persistence of external chloride and DIDS binding after chemical modification of Glu-681 in human band 3.
Am. J. Physiol. 277(4 Pt 1) , C791-9, (1999) Although its primary function is monovalent anion exchange, the band 3 protein also cotransports divalent anions together with protons at low pH. The putative proton binding site, Glu-681 in human erythrocyte band 3, is conserved throughout the anion exchange... |
|
|
Woodward's reagent K inactivation of Escherichia coli L-threonine dehydrogenase: increased absorbance at 340-350 nm is due to modification of cysteine and histidine residues, not aspartate or glutamate carboxyl groups.
Protein Sci. 5(2) , 382-90, (1996) L-Threonine dehydrogenase (TDH) from Escherichia coli is rapidly inactivated and develops a new absorbance peak at 347 nm when incubated with N-ethyl-5-phenylisoxazolium-3'-sulfonate (Woodward's reagent K, WRK). The cofactors, NAD+ or NADH (1.5 mM), provide c... |
|
|
Acidic residue modifications restore chaperone activity of β-casein interacting with lysozyme.
Int. J. Biol. Macromol. 49(4) , 616-21, (2011) An important factor in medicine and related industries is the use of chaperones to reduce protein aggregation. Here we show that chaperone ability is induced in β-casein by modification of its acidic residues using Woodward's Reagent K (WRK). Lysozyme at pH 7... |
|
|
A novel method for chemical modification of functional groups other than a carboxyl group in proteins by N-ethyl-5-phenylisooxazolium-3'-sulfonate (Woodward's reagent-K): inhibition of ADP-induced platelet responses involves covalent modification of aggregin, an ADP receptor.
Anal. Biochem. 240(2) , 251-61, (1996) The chemical reaction of N-ethyl-5-phenylisooxazolium-3'-sulfonate (Woodward's Reagent-K, WR-K) with a carboxyl group yields an enol ester that cannot be reduced by sodium borohydride in an aqueous solution, while other nucleophiles such as sulfhydryl, hydrox... |
|
|
Evidence for essential histidine and dicarboxylic amino-acid residues in the active site of UDP-glucose : solasodine glucosyltransferase from eggplant leaves.
Acta Biochim. Pol. 50(2) , 567-72, (2003) Effects of several chemical probes selectively modifying various amino-acid residues on the activity of UDP-glucose : solasodine glucosyltransferase from eggplant leaves was studied. It was shown that diethylpyrocarbonate (DEPC), a specific modifier of histid... |
|
|
The action of carboxyl modifying reagents on the ryanodine receptor/Ca2+ release channel of skeletal muscle sarcoplasmic reticulum.
Mol. Membr. Biol. 13(2) , 85-93, (1996) In this work we show that ryanodine binding to junctional sarcoplasmic reticulum (SR) membranes or purified ryanodine receptor (RyR) is inhibited in a time- and concentration-dependent fashion by prior treatment with the carboxyl reagent dicyclohexylcarbodiim... |
|
|
Modification of maize NADP-malic enzyme by Woodward's reagent 'K'.
Indian J. Biochem. Biophys. 34(3) , 253-8, (1997) Maize leaf NADP-malic enzyme was rapidly inactivated by micromolar concentrations of Woodward's reagent K (WRK). The inactivation followed pseudo-first order reaction kinetics. The order of reaction with respect to WRK was 1, suggesting that inactivation was ... |