Antimicrobial Agents and Chemotherapy
2007-06-01
Inhibitory activities of three classes of acyclic nucleoside phosphonates against murine polyomavirus and primate simian virus 40 strains.
Ilya Lebeau, Graciela Andrei, Marcela Krecmerová, Erik De Clercq, Antonin Holy, Robert Snoeck
Index: Antimicrob. Agents Chemother. 51(6) , 2268-73, (2007)
Full Text: HTML
Abstract
Murine polyomavirus and simian virus 40 were used to evaluate the potencies of the compounds of three classes of acyclic nucleoside phosphonates: (i) the original HPMP (3-hydroxy-2-phosphonomethoxypropyl) and PME (2-phosphonomethoxyethyl) derivatives, (ii) the 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidine (DAPy) derivatives, and (iii) a new class of HPMP derivatives containing a 5-azacytosine moiety. The last class showed the highest activities and selectivities against both polyomaviruses.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
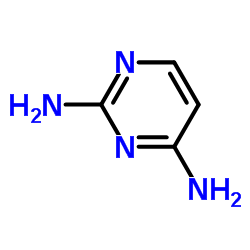 |
2,4-Diaminopyrimidine
CAS:156-81-0 |
C4H6N4 |
Related Articles:
More...
|
Contribution of nitric oxide-dependent guanylate cyclase and...
2015-12-01 [Neuropharmacology 99 , 422-31, (2015)] |
|
Design and optimization of hybrid of 2,4-diaminopyrimidine a...
2015-01-01 [Eur. J. Med. Chem. 96 , 269-80, (2015)] |
|
Cocrystals of 6-methyl-2-thiouracil: presence of the accepto...
2015-03-01 [Acta Crystallogr. C Struct. Chem. 71(Pt 3) , 229-38, (2015)] |
|
Ultra performance liquid chromatography tandem mass spectrom...
2009-03-01 [Anal. Bioanal. Chem 393(6-7) , 1709-18, (2009)] |
|
The acyclic 2,4-diaminopyrimidine nucleoside phosphonate act...
2010-04-16 [J. Biol. Chem. 285(16) , 12101-8, (2010)] |