[Additional effect of propiverine for naftopidil-resistant nocturia in the patient with benign prostate hypertrophy].
Noritaka Kamimura, Shingo Hatakeyama, Shigemasa Kudo, Takahiro Yoneyama, Yasuhiro Hashimoto, Takuya Koie, Kazuaki Yoshikawa, Toshiaki Kawaguchi, Shinya Takahashi, Chikara Ohyama
Index: Hinyokika Kiyo. 57(2) , 71-6, (2011)
Full Text: HTML
Abstract
The efficacy and safety of additional administration of propiverine were prospectively studied for naftopidil-resistant nocturia in patients with benign prostatic hypertrophy (BPH). Patients of 50 years and over with BPH who experienced nocturia twice a night or more and an overall International Prostate Symptom Score (IPSS) of 8 or more were first administered naftopidil (50 or 75 mg/day) for 4 weeks. Thirty subjects who did not show improvement in nocturia and requested further treatment were enrolled in the present study. Propiverine was then administered concomitantly 10 mg/day for 8 weeks. Significant improvement was observed with additional propiverine in the frequency of nocturia on voiding diary, total IPSS, voiding symptom, storage symptom and nocturnal voiding scores. No significant change was observed in the peak urinary flow rate (Qmax), mean urinary flow rate (Qave), voided urine volume, or residual urine volume. Adverse events were dysuria (2 cases), increased residual urine (6 cases), weak urine flow (1 case), thirsty (2 cases), angular cheilitis (1 case). Administration of propiverine was suspended in 7 subjects, 1 following dysuria and 6 following increased residual urine volume. The suspension of propiverine following increased residual urine volume was significantly more prevalent in subjects with pretreatment Qmax values of less than 10 ml/second or in subjects whose prostate specific antigen (PSA) levels were 2 ng/ml or more. In conclusion, the results indicate that additional administration of propiverine may be useful for the patients with BPH who have naftopidil-resistant nocturia. However, caution must be exercised regarding Qmax and PSA levels.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
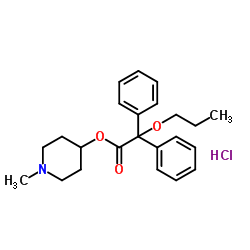 |
Propiverine hydrochloride
CAS:54556-98-8 |
C23H30ClNO3 |
|
Excitatory effect of propiverine hydrochloride on urethral a...
2012-06-01 [Int. J. Urol. 19(6) , 575-82, (2012)] |
|
An overview on mixed action drugs for the treatment of overa...
2012-01-01 [Urol. Int. 89(3) , 259-69, (2012)] |
|
Propiverine: a review of its use in the treatment of adults ...
2013-01-01 [Clin. Drug Investig. 33(1) , 71-91, (2013)] |
|
Efficacy of propiverine, an anticholinergic agent, in young ...
2011-09-26 [Life Sci. 89(13-14) , 456-9, (2011)] |
|
Oxybutynin and propiverine suppress adenosine triphosphate-i...
2010-08-01 [Urology 76(2) , 509.e8-12, (2010)] |