| Structure | Name/CAS No. | Articles |
|---|---|---|
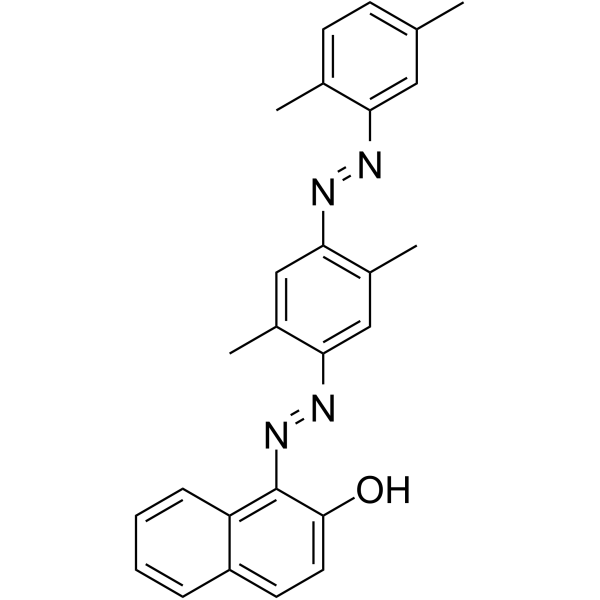 |
Oil Red O
CAS:1320-06-5 |
|
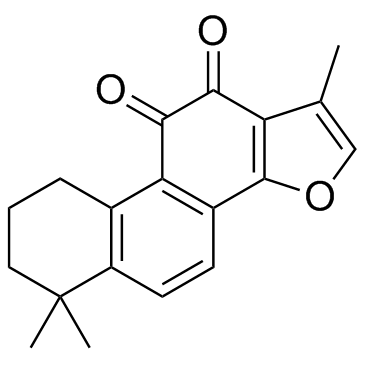 |
Tanshinone IIA
CAS:568-72-9 |
|
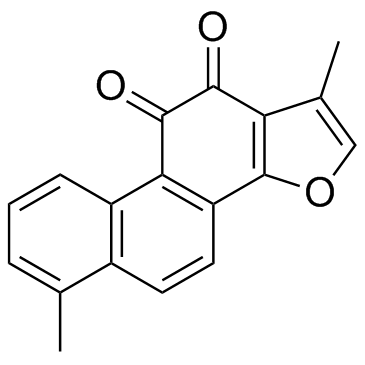 |
Tanshinone I
CAS:568-73-0 |