Study on the absolute configurations of 3-alkylphthalides using TDDFT calculations of chiroptical properties.
Li Li, Yi-Kang Si
Index: Chirality 24(12) , 987-93, (2012)
Full Text: HTML
Abstract
Absolute configurations (ACs) of 3-alkylphthalides including natural products (-)-3-n-butylphthalide ((-)-1) and fuscinarin have been studied using chiroptical properties and quantum chemical calculation. Electronic circular dichroism and optical rotatory dispersion spectra of (S)-1 predicted adopting time-dependent density functional theory and hybrid functionals coincide very well with the experimental and literature data of (-)-1, leading unambiguously to AC assignment as S for levorotatory isomer. The relationship between structures and chiroptical properties of 3-alkylphthalides were also studied using theoretical calculation. It is found that when the alkyl group is adjacent to the single chiral center in the molecule, both the length of the alkyl side chain and the polarity of solvent may exert significant effect on electronic circular dichroism spectra. On the basis of these observations, it is recommended that the long-chain alkyl group may be replaced by at least propyl instead of methyl group in such compounds. The present work shows that combination of chiroptical properties and ab initio calculations can provide a feasible and reliable way to the AC establishment of novel 3-alkylphthalide derivatives with a high degree of confidence.Copyright © 2012 Wiley Periodicals, Inc.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
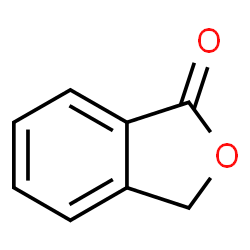 |
Phthalide
CAS:87-41-2 |
C8H6O2 |
|
Development of an Immunosensor for Determination of the Fung...
2015-07-22 [J. Agric. Food Chem. 63 , 6325-30, (2015)] |
|
Palladium-catalyzed cascade aryl addition/intramolecular lac...
2010-09-03 [J. Org. Chem. 75(17) , 6043-5, (2010)] |
|
Design and synthesis of marine fungal phthalide derivatives ...
2012-08-15 [Bioorg. Med. Chem. 20(16) , 4954-61, (2012)] |
|
Phthalide derivatives with antifungal activities against the...
2011-11-01 [J. Antibiot. 64(11) , 723-7, (2011)] |
|
Metabolic profiling of GuanXin II prescription based on meta...
2011-03-25 [J. Pharm. Biomed. Anal. 54(4) , 789-98, (2011)] |