Phthalide
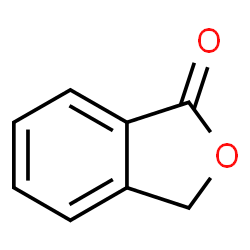
Phthalide structure
|
Common Name | Phthalide | ||
|---|---|---|---|---|
| CAS Number | 87-41-2 | Molecular Weight | 134.132 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 290.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H6O2 | Melting Point | 71-74 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 124.8±22.2 °C | |
Use of PhthalidePhthalide is a promising chemical scaffold with a potent anti-inflammatory efficacy. Phthalide can be used to synthesize a variety of phthalide derivatives including anti-inflammatory agent, antimicrobial, antioxidant[1][2][3]. |
| Name | 2-benzofuran-1(3H)-one |
|---|---|
| Synonym | More Synonyms |
| Description | Phthalide is a promising chemical scaffold with a potent anti-inflammatory efficacy. Phthalide can be used to synthesize a variety of phthalide derivatives including anti-inflammatory agent, antimicrobial, antioxidant[1][2][3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 290.0±0.0 °C at 760 mmHg |
| Melting Point | 71-74 °C(lit.) |
| Molecular Formula | C8H6O2 |
| Molecular Weight | 134.132 |
| Flash Point | 124.8±22.2 °C |
| Exact Mass | 134.036774 |
| PSA | 26.30000 |
| LogP | 0.99 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.585 |
| Water Solubility | sparingly soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TI3520000 |
| HS Code | 29322980 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Development of an Immunosensor for Determination of the Fungicide Chlorothalonil in Vegetables, Using Surface Plasmon Resonance.
J. Agric. Food Chem. 63 , 6325-30, (2015) An immunosensor based on surface plasmon resonance (SPR-sensor) was developed to analyze chlorothalonil residues and maximum residue limits (MRLs; 0.5-50 mg/kg) in vegetables in Japan. Conjugates of N... |
|
|
Palladium-catalyzed cascade aryl addition/intramolecular lactonization of phthalaldehyde to access 3-aryl- and alkenylphthalides.
J. Org. Chem. 75(17) , 6043-5, (2010) A palladium-catalyzed addition of arylboronic acids to phthalaldehyde, followed by an intramolecular lactonization to access 3-substituted phthalides, is described. The procedure tolerates a series of... |
|
|
Design and synthesis of marine fungal phthalide derivatives as PPAR-γ agonists.
Bioorg. Med. Chem. 20(16) , 4954-61, (2012) On the basis of a marine fungal phthalide (paecilocin A) skeleton, we synthesized 20 analogs and evaluated them for peroxisome proliferator-activated receptor gamma (PPAR-γ) binding and activation. Am... |
| 1-Isobenzofuranone |
| Rabcide |
| EINECS 201-744-0 |
| 1(3H)-4,5,6,7-tetrachloroisobenzofuranone |
| Fthalide |
| 3-oxo-1,3-dihydro-isobenzofuran |
| MFCD00005906 |
| 5-17-10-00007 (Beilstein Handbook Reference) |
| 1(3H)-Isobenzofuranone |
| isobenzofuran-1-one |
| 1-Phthalanone |
| KF-32 |
| 2-Hydroxymethylbenzoic acid, γ-lactone |
| PHH |
| 4,5,6,7-tetrachloro-1(3H)-isobenzofuranone |
| 4,5,6,7-Tetrachlorophthalidefthalide |
| 4,5,6,7-Tetrachlor-phthalid |
| Phthalide |
| 2-Benzofuran-1(3H)-one |
| 1H-Isobenzofuran-3-one |
| T56 BVO DHJ |
| 4,5,6,7-TETRACHLOROPHTHALIDE |
| 4,5,6,7-tetrachloroisobenzofuran-1(3H)-one |
| Isobenzofuranone |
| Phthalolactone |
 CAS#:6295-21-2
CAS#:6295-21-2 CAS#:95-47-6
CAS#:95-47-6 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:5159-41-1
CAS#:5159-41-1 CAS#:70744-47-7
CAS#:70744-47-7![[2-(hydroxymethyl)phenyl] trifluoromethanesulfonate Structure](https://image.chemsrc.com/caspic/092/112533-09-2.png) CAS#:112533-09-2
CAS#:112533-09-2 CAS#:612-14-6
CAS#:612-14-6 CAS#:18982-54-2
CAS#:18982-54-2 CAS#:92804-32-5
CAS#:92804-32-5 CAS#:118-90-1
CAS#:118-90-1 CAS#:108475-90-7
CAS#:108475-90-7 CAS#:55453-87-7
CAS#:55453-87-7 CAS#:5388-42-1
CAS#:5388-42-1 CAS#:57319-65-0
CAS#:57319-65-0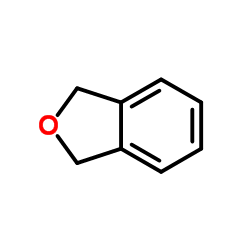 CAS#:496-14-0
CAS#:496-14-0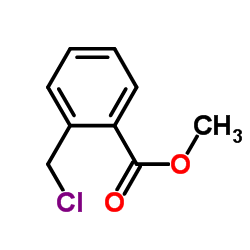 CAS#:34040-62-5
CAS#:34040-62-5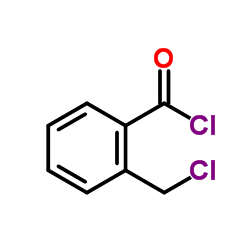 CAS#:42908-86-1
CAS#:42908-86-1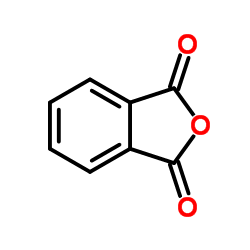 CAS#:85-44-9
CAS#:85-44-9 CAS#:55453-89-9
CAS#:55453-89-9 CAS#:40819-28-1
CAS#:40819-28-1
