Stereoselective synthesis of hydroxy stilbenoids and styrenes by atom-efficient olefination with thiophthalides.
Prithiba Mitra, Brateen Shome, Saroj Ranjan De, Anindya Sarkar, Dipakranjan Mal
Index: Org. Biomol. Chem. 10(14) , 2742-52, (2012)
Full Text: HTML
Abstract
The synthesis of stilbenoids and styryl carboxylic acids is accomplished with high E-stereoselectivity by olefination of aldehydes with thiophthalides under basic conditions. The olefination is highly atom-efficient as it only loses elemental sulfur during the reaction. This olefination, in conjunction with retro Kolbe-Schmitt reaction, allows facile synthesis of E-hydroxystilbenoids with minimal employment of protecting groups. This study also discloses two important findings: formation of i) 4-methylsulfanyl isocoumarins and ii) an 2-arylindenone.This journal is © The Royal Society of Chemistry 2012
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
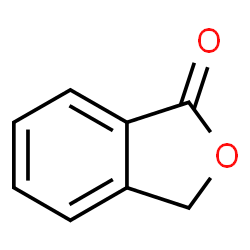 |
Phthalide
CAS:87-41-2 |
C8H6O2 |
|
Development of an Immunosensor for Determination of the Fung...
2015-07-22 [J. Agric. Food Chem. 63 , 6325-30, (2015)] |
|
Palladium-catalyzed cascade aryl addition/intramolecular lac...
2010-09-03 [J. Org. Chem. 75(17) , 6043-5, (2010)] |
|
Design and synthesis of marine fungal phthalide derivatives ...
2012-08-15 [Bioorg. Med. Chem. 20(16) , 4954-61, (2012)] |
|
Phthalide derivatives with antifungal activities against the...
2011-11-01 [J. Antibiot. 64(11) , 723-7, (2011)] |
|
Metabolic profiling of GuanXin II prescription based on meta...
2011-03-25 [J. Pharm. Biomed. Anal. 54(4) , 789-98, (2011)] |