Cyclopropylamines from N,N-dialkylcarboxamides and Grignard reagents in the presence of titanium tetraisopropoxide or methyltitanium triisopropoxide.
Armin de Meijere, Vladimir Chaplinski, Harald Winsel, Markus Kordes, Björn Stecker, Vesta Gazizova, Andrei I Savchenko, Roland Boese, Farina Schill
Index: Chemistry 16 , 13862-13875, (2010)
Full Text: HTML
Abstract
Thirty-three different N,N-dialkyl- and N-alkyl-N-phosphorylalkyl-substituted carboxamides 9-17 were treated with unsubstituted as well as with 2-alkyl-, 2,2-dialkyl-, and 3-alkenyl-substituted ethylmagnesium bromides 6 in the presence of stoichiometric amounts of titanium tetraisopropoxide or methyltitanium triisopropoxide to furnish substituted cyclopropylamines 20-25 in 20-98% yield, depending on the substituents with no (1:1) to excellent (>25:1) diastereoselectivities. Generally higher yields (up to 98%) of the cyclopropylamines 20-28 without loss of the diastereoselectivity were obtained with methyltitanium triisopropoxide as the titanium mediator. Under these conditions, even dioxolane-protected ketones and halogen-substituted and chiral as well as achiral alkyloxyalkyl-substituted carboxamides could be converted to the correspondingly substituted cyclopropylamines with unsubstituted as well as phenyl- and a variety of alkyl-substituted ethylmagnesium bromides in addition to numerous heteroatom-containing (e.g., halogen-, trityloxy-, tetrahydropyranyloxy-substituted) Grignard reagents (62 examples altogether). The transformation of N,N-diformylalkylamines 54 with ethylmagnesium bromide in the presence of methyltitanium triisopropoxide to N,N-dicyclopropyl-N-alkylamines 55 can be brought about in up to 82% yield (6 examples). An asymmetric variant of the titanium-mediated cyclopropanation of N,N-dialkylcarboxamides has been developed by applying chiral titanium mediators generated from stoichiometric amounts of titanium tetraisopropoxide and chiral diamino or diol ligands, respectively. The most efficient chiral mediators turned out to be titanium bistaddolates that provided the corresponding cyclopropylamines with enantiomeric excesses (ee) of up to 84%. Evaluation of several silyl-based additives revealed that the reaction can also efficiently be carried out with substoichiometric amounts (down to 25 mol%) of the titanium reagent, as long as 2-aryl- or 2-ethenyl-substituted ethylmagnesium halides are used and a concomitant slight decrease in yields is accepted. The newly developed methodology was successfully applied for the preparation of analogues with cyclopropylamine moieties of known drugs and natural products such as the nicotine metabolite (S)-Cotinine as well as the insecticides Dinotefuran and Imidacloprid.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
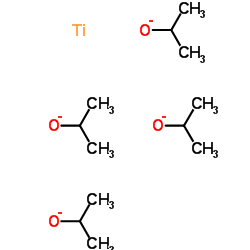 |
Titanium(4+) tetrapropan-2-olate
CAS:546-68-9 |
C12H28O4Ti | |
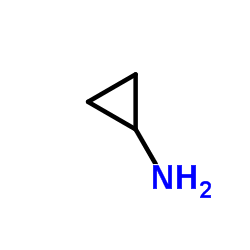 |
Cyclopropanamine
CAS:765-30-0 |
C3H7N |
|
Chirality of Single-Handed Twisted Titania Tubular Nanoribbo...
2015-08-01 [Chirality 27 , 543-50, (2015)] |
|
Surface properties of nanocrystalline TiO2 coatings in relat...
2015-08-01 [Biomed. Mater. 10 , 045012, (2015)] |
|
Thermally induced structural evolution and performance of me...
2014-05-27 [ACS Nano 8(5) , 4730-9, (2014)] |
|
Synthesis and electrochemical study of a hybrid structure ba...
2014-09-12 [Nanotechnology 25(36) , 365701, (2014)] |
|
UV-curable nanocomposite based on methacrylic-siloxane resin...
2015-07-22 [ACS Appl. Mater. Interfaces 7 , 15494-505, (2015)] |