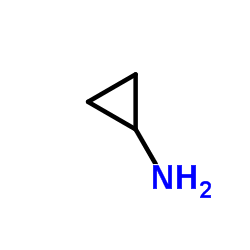
Cyclopropanamine
Cyclopropanamine structure
|
Common Name | Cyclopropanamine | ||
|---|---|---|---|---|
| CAS Number | 765-30-0 | Molecular Weight | 57.094 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 49.3±8.0 °C at 760 mmHg | |
| Molecular Formula | C3H7N | Melting Point | -50 °C | |
| MSDS | Chinese USA | Flash Point | -25.6±0.0 °C | |
| Symbol |



GHS02, GHS05, GHS07 |
Signal Word | Danger | |
|
Synthesis and antibacterial activity of some novel 4-oxopyrido[2,3-a]phenothiazines.
Arch. Pharm. (Weinheim) 347(11) , 861-72, (2014) A series of substituted 4-oxopyrido[2,3-a]phenothiazine-3-carboxylic acids (6a-d) were prepared via cyclization of the corresponding ethyl 7-(arylthioxy)-8-nitro(or azido)-4-oxoquinoline-3-carboxylates (3a-d/4a-d), followed by hydrolysis of the resultant este... |
|
|
Cyclopropylamines from N,N-dialkylcarboxamides and Grignard reagents in the presence of titanium tetraisopropoxide or methyltitanium triisopropoxide.
Chemistry 16 , 13862-13875, (2010) Thirty-three different N,N-dialkyl- and N-alkyl-N-phosphorylalkyl-substituted carboxamides 9-17 were treated with unsubstituted as well as with 2-alkyl-, 2,2-dialkyl-, and 3-alkenyl-substituted ethylmagnesium bromides 6 in the presence of stoichiometric amoun... |
|
|
Cyclopropylamine inactivation of cytochromes P450: role of metabolic intermediate complexes.
Arch. Biochem. Biophys. 436(2) , 265-75, (2005) The inactivation of cytochrome P450 enzymes by cyclopropylamines has been attributed to a mechanism involving initial one-electron oxidation at nitrogen followed by scission of the cyclopropane ring leading to covalent modification of the enzyme. Herein, we r... |
|
|
The Kulinkovich reaction in the synthesis of constrained n,n-dialkyl neurotransmitter analogues.
Org. Lett. 9(10) , 1987-90, (2007) An intermolecular Ti(IV)-mediated cyclopropanation reaction has been used to synthesize substituted 2-phenylcyclopropylamines and constrained analogues of the neurotransmitters histamine and tryptamine. Many hydroxy- and methoxy-substituted phenylcyclopropyla... |
|
|
Mutation of surface cysteine 374 to alanine in monoamine oxidase A alters substrate turnover and inactivation by cyclopropylamines.
Bioorg. Med. Chem. 13(10) , 3487-95, (2005) Modification of cysteine (Cys) residues inactivates monoamine oxidases (MAO) yet the crystal structure shows no conserved cysteines in the active site of MAO A (Ma, J. et al. J. Mol. Biol.2004, 338, 103-114). MAO A cysteine 374 was mutated to alanine and the ... |
|
|
Scalable synthesis of a prostaglandin EP4 receptor antagonist.
J. Org. Chem. 75(12) , 4078-85, (2010) The evolution of scalable, economically viable synthetic approaches to the potent and selective prostaglandin EP4 antagonist 1 is presented. The chromatography-free synthesis of multikilogram quantities of 1 using a seven-step sequence (six in the longest lin... |
|
|
Synthesis and biological activities of vitamin D-like inhibitors of CYP24 hydroxylase.
Steroids 77(3) , 212-23, (2012) Selective inhibitors of CYP24A1 represent an important synthetic target in a search for novel vitamin D compounds of therapeutic value. In the present work, we show the synthesis and biological properties of two novel side chain modified 2-methylene-19-nor-1,... |
|
|
Fluorinated phenylcyclopropylamines as inhibitors of monoamine oxidases.
ChemBioChem. 5(8) , 1033-43, (2004)
|
|
|
Fluorinated phenylcyclopropylamines. Part 4: effects of aryl substituents and stereochemistry on the inhibition of monoamine oxidases by 1-aryl-2-fluoro-cyclopropylamines.
Bioorg. Med. Chem. 13(7) , 2489-99, (2005) A series of para-ring-substituted (E)- and (Z)-1-aryl-2-fluorocyclopropylamines were examined as inhibitors of recombinant human liver monoamine oxidase A (MAO A) and B (MAO B). Unlike the parent 1-phenylcyclopropylamine, which is a selective inhibitor of MAO... |
|
|
Synthesis and properties of gem-(difluorocyclopropyl)amine derivatives of bicyclo[n.1.0]alkanes.
Org. Lett. 6(25) , 4767-70, (2004) [reaction: see text] Generation of difluorocarbene(carbenoid) in the presence of enamines derived from cyclic ketones results in overall insertion of CF2 to produce bicyclic difluorocyclopropylamines. These adducts are very weakly basic, and their thermal sta... |