| Structure | Name/CAS No. | Articles |
|---|---|---|
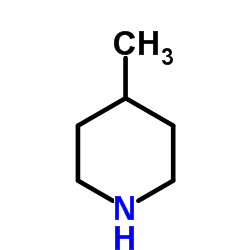 |
4-Pipecoline
CAS:626-58-4 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
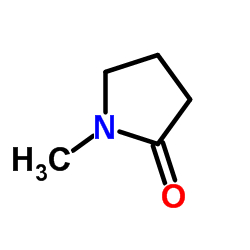 |
N-Methylpyrrolidone
CAS:872-50-4 |
|
 |
N,N′-diisopropylcarbodiimide
CAS:693-13-0 |