Citraconylation--a simple method for high protein sequence coverage in MALDI-TOF mass spectrometry.
Vojtech Kadlík, Martin Strohalm, Milan Kodícek
Index: Biochem. Biophys. Res. Commun. 305(4) , 1091-3, (2003)
Full Text: HTML
Abstract
Lysine epsilon -amino group reacts with citraconic anhydride forming a derivative, which is stable on terms for trypsin cleavage. This modification changes the spectrum of peptides formed by the trypsin action; as the number of trypsin-sensitive sites is reduced, the peptides with higher molecular mass can survive in the digest. The various studies of proteins by MALDI-TOF mass spectrometry are often complicated by the low sequence coverage of the peptide chain. This paper demonstrates that the modification of proteins by citraconylation before trypsin cleavage represents a simple experimental technique, which allows a significant increase of sequence coverage in MALDI-TOF mass spectrometry. This improvement is caused both by change of trypsin fragmentation pattern and by disturbance of the protein's native tertiary structure.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
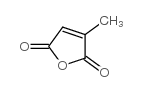 |
Citraconic anhydride
CAS:616-02-4 |
C5H4O3 |
|
MALDI imaging mass spectrometry profiling of N-glycans in fo...
2014-01-01 [PLoS ONE 9(9) , e106255, (2014)] |
|
Polymeric micelles with citraconic amide as pH-sensitive bon...
2014-08-25 [Int. J. Pharm. 471(1-2) , 28-36, (2014)] |
|
Pre-cultivation of adipose tissue-derived microvascular frag...
2015-01-01 [Eur. Cell. Mater. 29 , 190-200; discussion 200-1, (2015)] |
|
Experimental colitis in SIV-uninfected rhesus macaques recap...
2015-01-01 [Nat. Commun. 6 , 8020, (2015)] |
|
Dual-targeting and pH/redox-responsive multi-layered nanocom...
2015-08-01 [Biomaterials 60 , 42-52, (2015)] |