| Structure | Name/CAS No. | Articles |
|---|---|---|
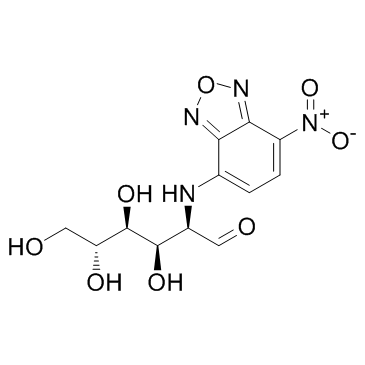 |
2-NBDG
CAS:186689-07-6 |
|
 |
5-TAMRA-SE
CAS:150810-68-7 |
|
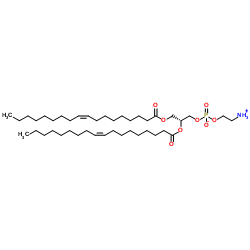 |
DOPE
CAS:4004-05-1 |
|
 |
5-TAMRA
CAS:91809-66-4 |