| Structure | Name/CAS No. | Articles |
|---|---|---|
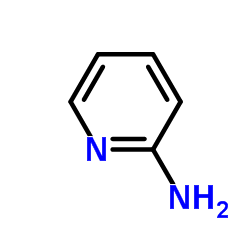 |
2-Aminopyridine
CAS:504-29-0 |
|
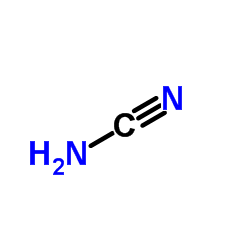 |
Cyanamide
CAS:420-04-2 |
|
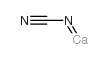 |
Calcium cyanamide
CAS:156-62-7 |