An efficient and regiospecific strategy to N7-substituted purines and its application to a library of trisubstituted purines.
Jinglin Liu, Qun Dang, Zhonglin Wei, Fuqiang Shi, Xu Bai
Index: J. Comb. Chem. 8(3) , 410-6, (2006)
Full Text: HTML
Abstract
A regiospecific strategy for the preparation of N(7)-substituted purines in an efficient manner was devised. This approach to 6,7,8-trisubstituted purines relies on the cyclization reactions of suitably substituted pyrimidines (1) with either a carboxylic acid or an aldehyde. The method development for the five-step synthetic strategy outlined here was completed using 5-amino-4,6-dichloropyrimidine (4) as the starting material. The utility of this methodology was demonstrated through the preparation of a 40-membered library of 6,7,8-trisubstituted purines (3) in good yields and high purity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
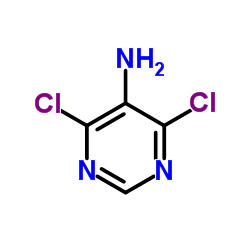 |
4,6-Dichloro-5-pyrimidinamine
CAS:5413-85-4 |
C4H3Cl2N3 |
|
6-(Alkylamino)-9-benzyl-9H-purines. A new class of anticonvu...
1988-03-01 [J. Med. Chem. 31(3) , 606-12, (1988)] |
|
Adenosine deaminase inhibitors. Synthesis and biological eva...
1992-10-30 [J. Med. Chem. 35(22) , 4180-4, (1992)] |
|
Synthesis of oxepane ring containing monocyclic, conformatio...
2007-09-14 [J. Org. Chem. 72(19) , 7427-30, (2007)] |
|
Sequential ring-closing metathesis and nitrone cycloaddition...
2006-08-04 [J. Org. Chem. 71(16) , 5980-92, (2006)] |
|
[Tetrahedron Lett. 48 , 1489, (2007)] |