Synthesis of oxepane ring containing monocyclic, conformationally restricted bicyclic and spirocyclic nucleosides from D-glucose: a cycloaddition approach.
Subhankar Tripathi, Biswajit G Roy, Michael G B Drew, Basudeb Achari, Sukhendu B Mandal
Index: J. Org. Chem. 72(19) , 7427-30, (2007)
Full Text: HTML
Abstract
Carbohydrate-derived substrates having (i) C-5 nitrone and C-3-O-allyl, (ii) C-4 vinyl and a C-3-O-tethered nitrone, and (iii) C-5 nitrone and C-4-allyloxymethyl generated tetracyclic isoxazolidinooxepane/-pyran ring systems upon intramolecular nitrone cycloaddition reactions. Deprotection of the 1,2-acetonides of these derivatives followed by introduction of uracil base via Vorbrüggen reaction condition and cleavage of the isooxazolidine rings as well as of benzyl groups by transfer hydrogenolysis yielded an oxepane ring containing bicyclic and spirocyclic nucleosides. The corresponding oxepane based nucleoside analogues were prepared by cleavage of isoxazolidine and furanose rings, coupling of the generated amino functionalities with 5-amino-4,6-dichloropyrimidine, cyclization to purine rings, and finally aminolysis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
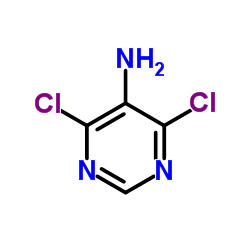 |
4,6-Dichloro-5-pyrimidinamine
CAS:5413-85-4 |
C4H3Cl2N3 |
|
6-(Alkylamino)-9-benzyl-9H-purines. A new class of anticonvu...
1988-03-01 [J. Med. Chem. 31(3) , 606-12, (1988)] |
|
An efficient and regiospecific strategy to N7-substituted pu...
2006-01-01 [J. Comb. Chem. 8(3) , 410-6, (2006)] |
|
Adenosine deaminase inhibitors. Synthesis and biological eva...
1992-10-30 [J. Med. Chem. 35(22) , 4180-4, (1992)] |
|
Sequential ring-closing metathesis and nitrone cycloaddition...
2006-08-04 [J. Org. Chem. 71(16) , 5980-92, (2006)] |
|
[Tetrahedron Lett. 48 , 1489, (2007)] |