| Structure | Name/CAS No. | Articles |
|---|---|---|
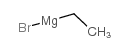 |
ETHYLMAGNESIUM BROMIDE
CAS:925-90-6 |
|
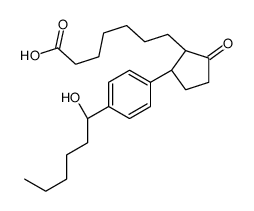 |
AH13205
CAS:148436-63-9 |