| Structure | Name/CAS No. | Articles |
|---|---|---|
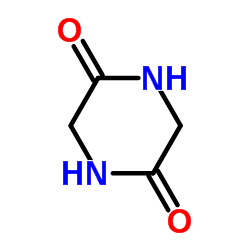 |
Glycine Anhydride
CAS:106-57-0 |
|
 |
Trichostatin A
CAS:58880-19-6 |