Trichostatin A

Trichostatin A structure
|
Common Name | Trichostatin A | ||
|---|---|---|---|---|
| CAS Number | 58880-19-6 | Molecular Weight | 302.368 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C17H22N2O3 | Melting Point | 141-143ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Trichostatin ATrichostatin A is a potent and specific inhibitor of HDAC class I/II, with an IC50 value of 1.8 nM for HDAC. |
| Name | trichostatin A |
|---|---|
| Synonym | More Synonyms |
| Description | Trichostatin A is a potent and specific inhibitor of HDAC class I/II, with an IC50 value of 1.8 nM for HDAC. |
|---|---|
| Related Catalog | |
| Target |
HDAC:1.8 nM (IC50) |
| In Vitro | Trichostatin A is a potent and specific inhibitor of HDAC class I/II, with an IC50 value of 1.8 nM for HDAC. Trichostatin A (TSA) inhibits proliferation of eight breast carcinoma cell lines with mean±SD IC50 of 124.4±120.4 nM (range, 26.4-308.1 nM). HDAC inhibitory activity of Trichostatin A is similar in all cell lines with mean IC50 of 2.4±0.5 nM (range, 1.5-2.9 nM)[1]. Trichostatin A (330 nM) increases Gαs protein expression in human myometrial cells, but does not increase Gαs mRNA levels[2]. Trichostatin A (20-75 nM) induces minimal cytotoxicity to adipose-derived stem cells (ADSCs), and enhances the osteogenic differentiation capacity of ADSCs[3]. In addition, Trichostatin A (0, 10, 100, 500 nM) dose-dependently decreases HDAC class I/II activity[4]. |
| In Vivo | Trichostatin A (500 μg/kg, s.c.) pronounces antitumor activity without causing any measurable toxicity in doses of up to 5 mg/kg by s.c. injection, in randomized controlled efficacy studies using the N-methyl-N-nitrosourea carcinogen-induced rat mammary carcinoma model[1]. |
| Cell Assay | Cells are cultured in a 96-well plate at 1×103 cells per well with 100 μL complete DMEM in the presence or absence of a HDAC inhibitor Trichostatin A for 72 h. Cytotoxicity is measured by performing WST-8 assay using a CCK-8 cell proliferation kit. The 450 nm absorbance is measured with a microplate reader. All experiments are carried out in triplicate and 3 independent experiments are performed[3]. |
| Animal Admin | Rats[1] Twelve rats are randomized to receive 500 μg/kg Trichostatin A in 50 μL DMSO, or 50 μL DMSO as vehicle control, by s.c. injection twice weekly for 4 weeks. In subsequent studies, 30 rats are randomized to receive Trichostatin A 500 μg/kg in 50 μL DMSO, or 50 μL DMSO as vehicle control, by s.c. injection daily for 4 weeks. Weekly tumor measurements, estimated tumor volumes, and body mass are recorded for each animal. Animals are sacrificed at the end of the 4-week study period; palpable tumors are resected and immediately snap-frozen in liquid nitrogen. Animals with tumors <2 cm in diameter or ulcerating tumors are withdrawn from study[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Melting Point | 141-143ºC |
| Molecular Formula | C17H22N2O3 |
| Molecular Weight | 302.368 |
| Exact Mass | 302.163055 |
| PSA | 69.64000 |
| LogP | 2.77 |
| Index of Refraction | 1.578 |
| InChIKey | RTKIYFITIVXBLE-WKWSCTOISA-N |
| SMILES | CC(C=CC(=O)NO)=CC(C)C(=O)c1ccc(N(C)C)cc1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332-H315-H317-H319-H335 |
| Precautionary Statements | P261-P280-P302 + P352 + P312-P304 + P340 + P312-P333 + P313 |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | 26-36 |
| RIDADR | NA 1993 / PGIII |
| WGK Germany | 3 |
| RTECS | MI5215000 |
| HS Code | 2924299090 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
A specific A/T polymorphism in Western tyrosine phosphorylation B-motifs regulates Helicobacter pylori CagA epithelial cell interactions.
PLoS Pathog. 11(2) , e1004621, (2015) Helicobacter pylori persistently colonizes the human stomach, with mixed roles in human health. The CagA protein, a key host-interaction factor, is translocated by a type IV secretion system into host... |
|
|
Preservative activity of lavender hydrosols in moisturizing body gels.
Lett. Appl. Microbiol. 60(1) , 27-32, (2014) The study was undertaken to verify the antimicrobial activity of Lavandula angustifolia hydrosols in moisturizing body gels. The inhibition efficacy of four lavender hydrosols (obtained from fresh or ... |
|
|
Nicotinamide exacerbates hypoxemia in ventilator-induced lung injury independent of neutrophil infiltration.
PLoS ONE 10(4) , e0123460, (2015) Ventilator-induced lung injury is a form of acute lung injury that develops in critically ill patients on mechanical ventilation and has a high degree of mortality. Nicotinamide phosphoribosyltransfer... |
| Antibiotic A-300 |
| 2,4-Heptadienamide, 7-[4-(dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-, (2E,4E,6R)- |
| (2E,4E,6R)-7-[4-(Dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide |
| (R)-Trichostatin A |
| 2,4-Heptadienamide, 7-[4-(dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-, (2E,4E,6R)- (9CI) |
| TSA |
| 2,4-Heptadienamide, 7-(4-(dimethylamino)phenyl)-N-hydroxy-4,6-dimethyl-7-oxo-, (2E,4E,6R)- |
| 2,4-Heptadienamide, 7-(4-(dimethylamino)phenyl)-N-hydroxy-4,6-dimethyl-7-oxo- |
| Trichostatin A |
| 7-(4-(Dimethylamino)phenyl)-N-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide |
| 7-[4-(dimethylamino)phenyl]-N-hydroxy-4,6R-dimethyl-7-oxo-2E,4E-heptadienamide |
| (2E,4E,6R)-7-[4-(Dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxohepta-2,4-dienamide |
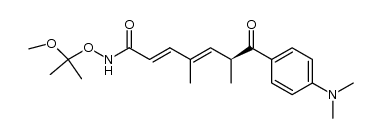 CAS#:122222-87-1
CAS#:122222-87-1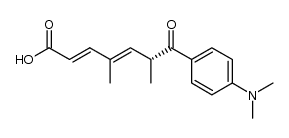 CAS#:114127-18-3
CAS#:114127-18-3![ethyl (2E,4E,6R,7R)-7-[4-(dimethylamino)phenyl]-7-hydroxy-4,6-dim ethyl-hepta-2,4-dienoate Structure](https://image.chemsrc.com/caspic/352/934246-98-7.png) CAS#:934246-98-7
CAS#:934246-98-7 CAS#:100-10-7
CAS#:100-10-7 CAS#:122222-78-0
CAS#:122222-78-0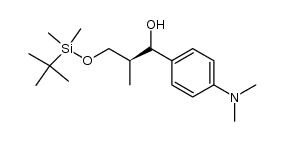 CAS#:122222-72-4
CAS#:122222-72-4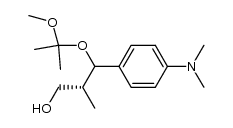 CAS#:122222-76-8
CAS#:122222-76-8 CAS#:122222-81-5
CAS#:122222-81-5 CAS#:114127-17-2
CAS#:114127-17-2