| Structure | Name/CAS No. | Articles |
|---|---|---|
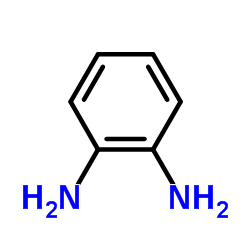 |
o-Phenylenediamine
CAS:95-54-5 |
|
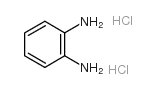 |
1,2-Benzenediamine,hydrochloride (1:2)
CAS:615-28-1 |
|
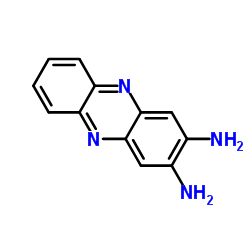 |
2,3-Diaminophenazine
CAS:655-86-7 |