Synthesis and antiviral activities of synthetic glutarimide derivatives.
Xing-yue Ji, Zhao-jin Zhong, Si-tu Xue, Shuai Meng, Wei-ying He, Rong-mei Gao, Yu-huan Li, Zhuo-rong Li
Index: Chem. Pharm. Bull. 58(11) , 1436-41, (2010)
Full Text: HTML
Abstract
A series of novel glutarimide compounds were synthesized and their antiviral activities were evaluated. The compounds displaying the strongest antiviral activities included 5, 6f, 7e and 9 against coxsackievirus B3 (Cox B3), 10 and 6f against influenza virus A (influenza A) and 7a against herpes simplex virus 2 (HSV-2). However, most of the synthetic glutarimides showed comparatively much weaker activity against influenza A, Cox B3 and HSV-2 than the natural glutarimide compounds tested. Based on the results, it seemed likely that a conjugated system at the β-substituted moiety provides stronger antiviral activity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
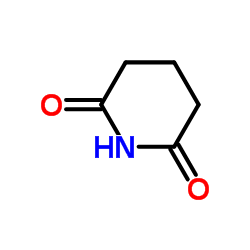 |
Glutarimide
CAS:1121-89-7 |
C5H7NO2 |
|
Comparative characterization of the lactimidomycin and iso-m...
2014-12-16 [Biochemistry 53(49) , 7854-65, (2014)] |
|
Iso-migrastatin congeners from Streptomyces platensis and ge...
2005-08-31 [J. Am. Chem. Soc. 127(34) , 11930-1, (2005)] |
|
Enantiomerization mechanism of thalidomide and the role of w...
2012-11-05 [Chemistry 18(45) , 14305-13, (2012)] |
|
Evaluation of new migrastatin and dorrigocin congeners unvei...
2008-11-15 [Bioorg. Med. Chem. Lett. 18(22) , 5951-4, (2008)] |
|
Inhibition of macrophage activation and suppression of graft...
2011-09-01 [Inflamm. Res. 60(9) , 879-88, (2011)] |