| Structure | Name/CAS No. | Articles |
|---|---|---|
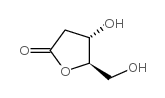 |
(4S,5R)-4-Hydroxy-5-(hydroxymethyl)dihydrofuran-2(3H)-one
CAS:34371-14-7 |
Cecilia Paris, Susana Encinas, Nourreddine Belmadoui, María J Climent, Miguel Angel Miranda
Index: Org. Lett. 10(20) , 4409-12, (2008)
Full Text: HTML
Photolysis of the title dyads under aerobic conditions leads to a 2-deoxyribonolactone derivative. Laser flash photolysis reveals that the process occurs from the short-lived benzophenone-like triplet excited state. A mechanism involving intramolecular electron transfer with the purine bases (adenine, guanine, or 8-oxoadenine) as donors is proposed.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
(4S,5R)-4-Hydroxy-5-(hydroxymethyl)dihydrofuran-2(3H)-one
CAS:34371-14-7 |
C5H8O4 |
|
Reduced repair capacity of a DNA clustered damage site compr...
2014-04-01 [Mutat. Res. Fundam. Mol. Mech. Mutagen. 762 , 32-9, (2014)] |
|
Analysis of base excision DNA repair of the oxidative lesion...
2006-01-01 [Meth. Enzymol. 408 , 48-64, (2006)] |
|
Histone-catalyzed cleavage of nucleosomal DNA containing 2-d...
2012-05-16 [J. Am. Chem. Soc. 134(19) , 8090-3, (2012)] |
|
Mutagenic effects of abasic and oxidized abasic lesions in S...
2005-01-01 [Nucleic Acids Res. 33(19) , 6196-202, (2005)] |
|
Use of fluorescence sensors to determine that 2-deoxyribonol...
2007-01-01 [Angew. Chem. Int. Ed. Engl. 46(4) , 561-4, (2007)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved