Phase II evaluation of bisantrene in acute leukemia. A Southwest Oncology Group Study.
G M Mills, S Dahlberg, J Cowan, B A Neilan, C H Gumbart, K Hussein, C A Coltman
Index: Am. J. Clin. Oncol. 12(6) , 507-10, (1989)
Full Text: HTML
Abstract
Twenty-nine patients with heavily pretreated acute leukemia in relapse were treated with bisantrene (maximum dose 120 mg/m2/day x 5) in a phase II study. Twenty-seven of the 29 patients were evaluable for response, receiving a total of 53 courses of treatment. There were three complete remissions (11%) lasting 27, 107, and 115 days. One brief partial remission of 43 days was also seen for a total response rate of 15%. Toxicity was mainly limited to the expected myelotoxicity with minimal nonhematologic toxicity seen. Although the complete remission rate is low, an antileukemic effect was seen in the majority of the patients treated. Sixty-one percent of the patients had at least a 50% decrease in the circulating blast count and 32% had at least a 50% decrease in the number of bone marrow blasts. We conclude that bisantrene does have an antileukemic effect, but that the optimal starting dose is not yet established.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
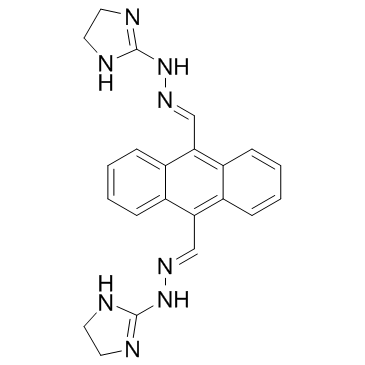 |
Bisantrene
CAS:78186-34-2 |
C22H22N8 |
|
In vitro effects of bisantrene on fresh clonogenic leukemia ...
1990-01-01 [Haematologica 75(6) , 527-31, (1990)] |
|
Phase II trial of Bisantrene for metastatic melanoma: an Ill...
1991-01-01 [Med. Pediatr. Oncol. 19(2) , 126-8, (1991)] |
|
Retroviral transfer of the human MDR1 gene confers resistanc...
2014-04-29 [Clin. Cancer Res. 2(6) , 973-80, (1996)] |
|
Synthesis, DNA-damaging and cytotoxic properties of novel to...
1998-01-20 [Bioorg. Med. Chem. Lett. 8(2) , 121-6, (1998)] |
|
Bisantrene in advanced, hormone-resistant carcinoma of the p...
1990-08-01 [Invest. New Drugs 8(3) , 313-5, (1990)] |