Bisantrene
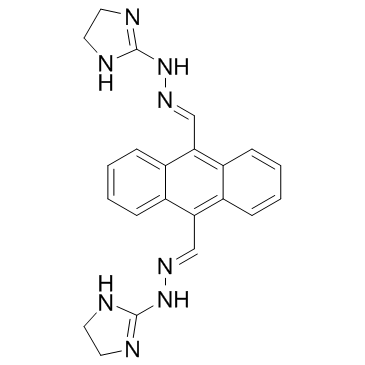
Bisantrene structure
|
Common Name | Bisantrene | ||
|---|---|---|---|---|
| CAS Number | 78186-34-2 | Molecular Weight | 398.46400 | |
| Density | 1.41g/cm3 | Boiling Point | 646.3ºC at 760 mmHg | |
| Molecular Formula | C22H22N8 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 344.7ºC | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
|
In vitro effects of bisantrene on fresh clonogenic leukemia cells: a preliminary study on 15 cases.
Haematologica 75(6) , 527-31, (1990) In vitro clonogenic assays may be useful for determining the sensitivity of leukemic cells to chemotherapeutic agents. We evaluated the antileukemic effect of Bisantrene (an anthracene derivative now undergoing phase II clinical trials in relapsed/resistant a... |
|
|
Phase II trial of Bisantrene for metastatic melanoma: an Illinois cancer council study.
Med. Pediatr. Oncol. 19(2) , 126-8, (1991) Sixteen patients with metastatic melanoma entered this phase II study of the efficacy of monthly cycles of Bisantrene. Toxicity was characterized by leukopenia, resulting in the hospitalization of one patient for a febrile incident, and superficial phlebitis.... |
|
|
Retroviral transfer of the human MDR1 gene confers resistance to bisantrene-specific hematotoxicity.
Clin. Cancer Res. 2(6) , 973-80, (1996) In this work, we demonstrate a protective effect conferred by the human multidrug resistance gene (MDR1) to populations of the murine hematopoietic system against the toxic effects of bisantrene, a novel intercalating cytotoxic agent under investigation as an... |
|
|
Synthesis, DNA-damaging and cytotoxic properties of novel topoisomerase II-directed bisantrene analogues.
Bioorg. Med. Chem. Lett. 8(2) , 121-6, (1998) New bisantrene analogues were synthesized, bearing one or two 4,5-dihydro-1H-imidazol-2-yl hydrazone side chains at positions 1,4 or 9 of the anthracene ring system. A 10-azabioisostere was also prepared. The position of substituents in structurally isomeric ... |
|
|
Bisantrene in advanced, hormone-resistant carcinoma of the prostate: an Illinois Cancer Council phase II study.
Invest. New Drugs 8(3) , 313-5, (1990)
|
|
|
Bisantrene in relapsed and refractory acute myelogenous leukemia.
Leuk. Lymphoma 9(3) , 217-20, (1993) Because of the lack of standard treatment in refractory and relapsed acute myelogenous leukemia (AML) several new drugs have been employed alone to evaluate their efficacy in this peculiar category of patient. Bisantrene, a new anthracene bishydrazone derivat... |
|
|
Phase II evaluation of bisantrene in refractory multiple myeloma. A Southwest Oncology Group study.
Invest. New Drugs 9(4) , 329-31, (1991)
|
|
|
Phase II evaluation of bisantrene in acute leukemia. A Southwest Oncology Group Study.
Am. J. Clin. Oncol. 12(6) , 507-10, (1989) Twenty-nine patients with heavily pretreated acute leukemia in relapse were treated with bisantrene (maximum dose 120 mg/m2/day x 5) in a phase II study. Twenty-seven of the 29 patients were evaluable for response, receiving a total of 53 courses of treatment... |
|
|
Effect of hypoxia and acidosis on the cytotoxicity of mitoxantrone, bisantrene and amsacrine and their platinum complexes at normal and hyperthermic temperatures.
Anticancer Res. 12(3) , 827-36, (1992) In an effort to synthesize drugs which would become much more cytotoxic at clinically achievable hyperthermic temperatures, complexes of the tetrachloro-platinum(II) dianion were made with two anthracene dye derivatives, MITOX and BISANT, and the acridine dye... |
|
|
Remarkable interference with telomeric function by a G-quadruplex selective bisantrene regioisomer
Biochem. Pharmacol. 79(12) , 1781-90, (2010) The use of small molecules able to induce and stabilize selected G-quadruplex arrangements can cause telomerase inhibition and telomere dysfunction in cancer cells, thus providing very selective therapeutic approaches. Effective stabilizers usually comprise a... |