Carbonic anhydrase inhibitors: Thioxolone versus sulfonamides for obtaining isozyme-selective inhibitors?
Alessio Innocenti, Alfonso Maresca, Andrea Scozzafava, Claudiu T. Supuran, Alessio Innocenti, Alfonso Maresca, Andrea Scozzafava, Claudiu T. Supuran
Index: Bioorg. Med. Chem. Lett. 18(14) , 3938-41, (2008)
Full Text: HTML
Abstract
Inhibition of 13 mammalian isoforms of the metalloenzyme carbonic anhydrase (CA, EC 4.2.1.1), CA I-XV, with thioxolone (6-hydroxy-1,3-benzoxathiol-2-one) and two sulfonamides was investigated. Thioxolone was inefficient for generating isozyme-selective inhibitors, since except for CA I which is inhibited in the nanomolar range (K(I) of 91 nM), the remaining 12 isoforms were inhibited with a very flat profile (K(I)s in the range of only 4.93-9.04 microM). In contrast to thioxolone, 3,5-dichloro-4-hydroxybenzenesulfonamide as well as the clinically used heterocyclic sulfonamide acetazolamide showed K(I)s in the range of 58 nM-78.6 microM and 2.5 nM-200 microM, respectively, against the 13 investigated mammalian CAs. The sulfonamide zinc-binding group is thus superior to the thiol one for generating CA inhibitors with a varied and sometimes isozyme-selective inhibition profile against the mammalian enzymes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
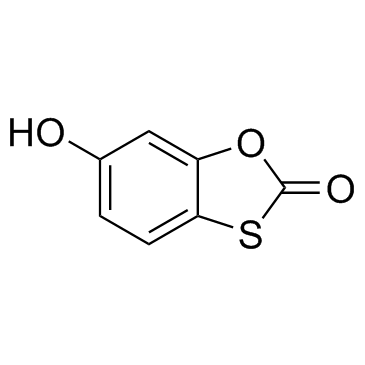 |
tioxolone
CAS:4991-65-5 |
C7H4O3S |
|
The supramolecular structure of 6-hydroxy-1,3-benzoxathiol-2...
2004-06-01 [Acta Crystallogr. C 60(Pt 6) , o395-6, (2004)] |
|
[Antimicrobial and cytostatic properties of 6-hydroxy-1,3-be...
1969-08-01 [Arzneimittelforschung 19(8) , 1298-304, (1969)] |
|
Contact dermatitis from thioxolone.
1993-08-01 [Contact Dermatitis 29(2) , 96, (1993)] |
|
Mechanism of allergic cross-reactions--I. Multispecific bind...
1991-06-01 [Mol. Immunol. 28(6) , 641-54, (1991)] |
|
Inhibition of carbonic anhydrase II by thioxolone: a mechani...
2008-03-11 [Biochemistry 47(10) , 3174-84, (2008)] |