| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(2S)-2-(Dibenzylamino)-1-propanol
CAS:60479-65-4 |
|
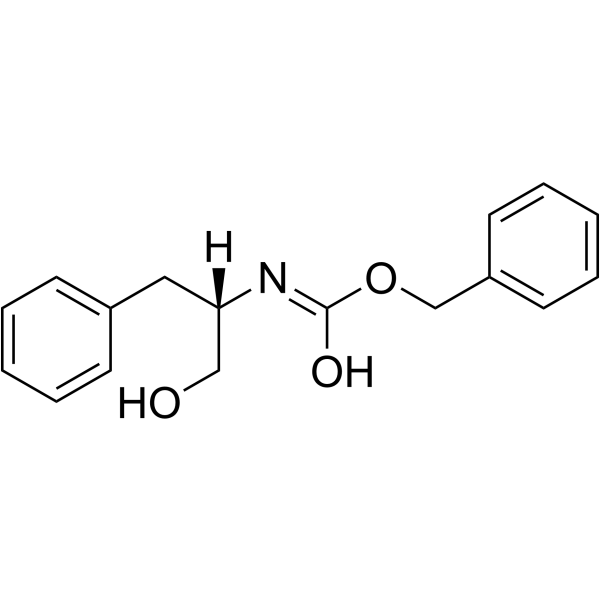 |
(S)-Cbz-Phenylalaninol
CAS:6372-14-1 |
|
 |
(S)-(+)-2-(Dibenzylamino)-3-phenyl-1-propanol
CAS:111060-52-7 |