(S)-Cbz-Phenylalaninol
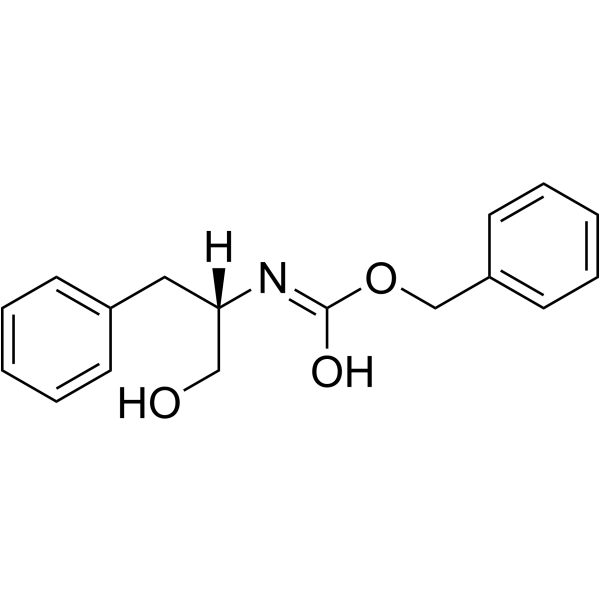
(S)-Cbz-Phenylalaninol structure
|
Common Name | (S)-Cbz-Phenylalaninol | ||
|---|---|---|---|---|
| CAS Number | 6372-14-1 | Molecular Weight | 285.338 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 489.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C17H19NO3 | Melting Point | 90-94ºC | |
| MSDS | Chinese USA | Flash Point | 249.6±28.7 °C | |
|
Preparation of Aminoalkyl Chlorohydrin Hydrochlorides: Key Building Blocks for Hydroxyethylamine-Based HIV Protease Inhibitors.
J. Org. Chem. 61 , 3635, (1996) Enantiomerically pure N,N-dibenzyl-alpha-amino aldehydes reacted with (chloromethyl)lithium, generated in situ from bromochloromethane and lithium metal, to give predominantly erythro aminoalkyl epoxides. Treatment of the crude epoxides with aqueous hydrochlo... |
|
|
Stereoselective Synthesis of HIV-1 Protease Inhibitor, DMP 323.
J. Org. Chem. 61 , 444, (1996) DMP 323, a potent HIV-1 protease inhibitor, has been synthesized by an efficient stereoselective process, amenable to large scale preparations. The core C(2) symmetric diol was synthesized by a stereoselective pinacol coupling of CBZ protected D-phenylalanine... |
|
|
Cyclic HIV protease inhibitors: synthesis, conformational analysis, P2/P2' structure-activity relationship, and molecular recognition of cyclic ureas.
J. Med. Chem. 39 , 3514, (1996) High-resolution X-ray structures of the complexes of HIV-1 protease (HIV-1PR) with peptidomimetic inhibitors reveal the presence of a structural water molecule which is hydrogen bonded to both the mobile flaps of the enzyme and the two carbonyls flanking the ... |
|
|
Inhibitors of HIV-1 proteinase containing 2-heterosubstituted 4-amino-3-hydroxy-5-phenylpentanoic acid: synthesis, enzyme inhibition, and antiviral activity.
J. Med. Chem. 37 , 3079, (1994) A convenient procedure for the synthesis of 2-heterosubstituted statine derivatives as novel building blocks in HIV-protease inhibitors has been developed. The synthesis starts with protected L-phenylalaninols, which were converted to gamma-amino alpha, beta-... |
|
|
Improved cyclic urea inhibitors of the HIV-1 protease: synthesis, potency, resistance profile, human pharmacokinetics and X-ray crystal structure of DMP 450.
Chem. Biol. 3 , 301, (1996) Effective HIV protease inhibitors must combine potency towards wild-type and mutant variants of HIV with oral bioavailability such that drug levels in relevant tissues continuously exceed that required for inhibition of virus replication. Computer-aided desig... |
|
|
Aldrichimica Acta 28 , 13, (1995)
|
|
|
Liu, C. et al.
Org. Process Res. Dev. 1 , 45, (1997)
|