Induction of mutation in mouse FM3A cells by N4-aminocytidine-mediated replicational errors.
M Takahashi, M Nishizawa, K Negishi, F Hanaoka, M A Yamada, H Hayatsu
Index: Mol. Cell. Biol. 8(1) , 347-52, (1988)
Full Text: HTML
Abstract
To explore the potential use of a nucleoside analog, N4-aminocytidine, in studies of cellular biology, the mechanism of mutation induced by this compound in mouse FM3A cells in culture was studied. On treatment of cells in suspension with N4-aminocytidine, the mutation to ouabain resistance was induced. The major DNA-replicating enzyme in mammalian cells, DNA polymerase alpha, was used to investigate whether the possible cellular metabolite of N4-aminocytidine, N4-aminodeoxycytidine 5'-triphosphate (dCamTP), can be incorporated into the DNA during replication. Using [3H]dCamTP in an in vitro DNA-synthesizing system, we were able to show that this nucleotide analog can be incorporated into newly formed DNA and that it can serve as a substitute for either dCTP or dTTP. dCamTP in the absence of dCTP maintained the activated calf thymus DNA-directed polymerization of deoxynucleoside triphosphates as efficiently as in its presence. Even in the presence of dCTP, dCamTP was incorporated into the polynucleotide. When dCamTP was used as a single substrate in the poly(dA)-oligo(dT)-directed polymerase reaction, it was incorporated into the polynucleotide fraction. The extent of incorporation was 4% of that of dTTP incorporation when dTTP was used as a single substrate. Even in the presence of dTTP, dCamTP incorporation was observed. A copolymer containing N4-aminocytosine residues was shown to incorporate guanine residues opposite the N4-aminocytosines. However, we were unable to observe adenine incorporation opposite N4-aminocytosine in templates. These cell-free experiments show that an AT-to-GC transition can take place in the presence of dCamTP during DNA synthesis, strongly suggesting that the mutation induced in the FM3A cells by N4-aminocytidine is due to replicational errors.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
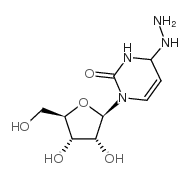 |
n4-aminocytidine
CAS:57294-74-3 |
C9H16N4O5 |
|
Genotoxic properties of representatives of alkylindazoles an...
2015-06-01 [Food Chem. Toxicol. 80 , 130-6, (2015)] |
|
Mutagenic nucleoside analog N4-aminocytidine: metabolism, in...
1988-11-01 [J. Bacteriol. 170(11) , 5257-62, (1988)] |
|
An improved synthesis of N4-aminocytidine.
1987-09-01 [Chem. Pharm. Bull. 35(9) , 3884-7, (1987)] |
|
[Molecular mechanism of N4-aminocytidine mutagenesis].
1990-05-01 [Yakugaku Zasshi 110(5) , 293-303, (1990)] |
|
Mutagenesis by N4-aminocytidine: induction of AT to GC trans...
1985-12-03 [Biochemistry 24 , 7273, (1985)] |