Inhibition of 2,3-oxidosqualene: beta-amyrin-cyclase, S-adenosyl-L-methionine: cycloartenol C-24-methyltransferase and cycloeucalenol: obtusifoliol isomerase by rationally designed molecules containing a tertiary amine function.
A Rahier, P Bouvier, L Cattel, A Narula, P Benveniste
Index: Biochem. Soc. Trans. 11(5) , 537-43, (1983)
Full Text: HTML
Abstract
25-Azacycloartanol (I), 2-aza-2-dihydrosqualene (II) and Tridemorph (2,6-dimethyl-N-tridecylmorpholine) (III) are potent inhibitors of higher plant sterol biosynthesis. The first two molecules have been designed using rational enzymological concepts. I, II and III were shown to inhibit the S-adenosyl-L-methionine: cycloartenol C-24-methyltransferase, the 2,3-oxidosqualene: beta-amyrin-cyclase and the cycloeucalenol: obtusifoliol isomerase, respectively. Inhibition was demonstrated either in vivo on bramble cell suspensions or in vitro on microsomes from maize seedlings. Each inhibitor has been shown to have a high affinity for its presumed enzymic target and only negligible inhibitory action on the other two enzymes. The applications of these results to further physiological studies are discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
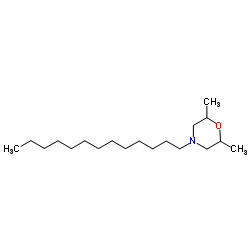 |
Tridemorph
CAS:24602-86-6 |
C19H39NO |
|
Effect of some sterol-biosynthesis-inhibiting fungicides on ...
1989-01-01 [Steroids 53(3-5) , 393-412, (1989)] |
|
[Teratogenic effect of the fungicide calixin].
1981-01-01 [Vopr. Pitan. (6) , 55-61, (1981)] |
|
Human lamin B receptor exhibits sterol C14-reductase activit...
1998-06-15 [Biochim. Biophys. Acta 1392(2-3) , 233-44, (1998)] |
|
Synthesis and structure revision of calyxin natural products...
2006-04-14 [J. Org. Chem. 71(8) , 3176-83, (2006)] |
|
Inhibition of microbial cholesterol oxidases by dimethylmorp...
1990-01-01 [J. Steroid Biochem. 35(1) , 107-13, (1990)] |