Tridemorph
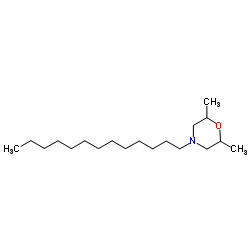
Tridemorph structure
|
Common Name | Tridemorph | ||
|---|---|---|---|---|
| CAS Number | 24602-86-6 | Molecular Weight | 297.519 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 371.3±17.0 °C at 760 mmHg | |
| Molecular Formula | C19H39NO | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 109.5±23.3 °C | |
| Symbol |



GHS07, GHS08, GHS09 |
Signal Word | Danger | |
|
Effect of some sterol-biosynthesis-inhibiting fungicides on the biosynthesis of polyisoprenoid compounds in barley seedings.
Steroids 53(3-5) , 393-412, (1989) The effect of five sterol-biosynthesis-inhibiting (SBI) fungicides, triadimefon, triarimol, diclobutrazol, tridemorph, and fenpropimorph on the germination, growth, and chloroplast pigment and sterol content of barley seedlings has been studied. Triadimefon, ... |
|
|
[Teratogenic effect of the fungicide calixin].
Vopr. Pitan. (6) , 55-61, (1981) Experimental studies on Wistar rats revealed that the fungicide calixin (N-tridecyl-2.6-dimethylmorpholine) has a teratogenic effect. This effect manifested in edemas, hemorrhages, hematomas, abnormal development of the brain (hydrocephalia), visceral cranium... |
|
|
Human lamin B receptor exhibits sterol C14-reductase activity in Saccharomyces cerevisiae.
Biochim. Biophys. Acta 1392(2-3) , 233-44, (1998) Lamin B receptor (LBR), a nuclear protein of avian and mammalian cells, contains an hydrophobic domain that shares extensive structural similarities with the members of the sterol reductase family. To test if the sterol-reductase-like domain of LBR could be e... |
|
|
Inhibition of 2,3-oxidosqualene: beta-amyrin-cyclase, S-adenosyl-L-methionine: cycloartenol C-24-methyltransferase and cycloeucalenol: obtusifoliol isomerase by rationally designed molecules containing a tertiary amine function.
Biochem. Soc. Trans. 11(5) , 537-43, (1983) 25-Azacycloartanol (I), 2-aza-2-dihydrosqualene (II) and Tridemorph (2,6-dimethyl-N-tridecylmorpholine) (III) are potent inhibitors of higher plant sterol biosynthesis. The first two molecules have been designed using rational enzymological concepts. I, II an... |
|
|
Synthesis and structure revision of calyxin natural products.
J. Org. Chem. 71(8) , 3176-83, (2006) Tandem Prins cyclization and Friedel-Crafts reaction with an electron-rich aromatic ring were used to prepare the core structures of calyxin natural products. The proposed structure of epicalyxin F was prepared and shown to be incorrect. Several calyxin natur... |
|
|
Inhibition of microbial cholesterol oxidases by dimethylmorpholines.
J. Steroid Biochem. 35(1) , 107-13, (1990) Cholesterol oxidase is a potentially important enzyme in steroid transformations, catalysing the conversion of 3-hydroxy-5-ene steroids to 3-keto-4-ene derivatives via a 3-keto-5-ene intermediate. Morpholine derivatives, especially fenpropimorph and tridemorp... |
|
|
Determination of tridemorph and other fungicide residues in fruit samples by liquid chromatography-electrospray tandem mass spectrometry.
J. Chromatogr. A. 1045(1-2) , 137-43, (2004) A rapid and sensitive liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS-MS) method for the determination of tridemorph and other pre- and post-harvest fungicides (carbendazim, thiabendazole, imazalil, propiconazole and bitertan... |
|
|
Sterol biosynthesis via cycloartenol and other biochemical features related to photosynthetic phyla in the amoeba Naegleria lovaniensis and Naegleria gruberi.
Eur. J. Biochem. 164(2) , 427-34, (1987) The sterols and sterol precursors of two amoebae of the genus Naegleria, Naegleria lovaniensis and Naegleria gruberi were investigated. Cycloartenol, the sterol precursor in photosynthetic organisms, is present in both amoebae. In N. lovaniesis, it is accompa... |
|
|
The action of the systemic fungicides tridemorph and fenpropimorph on sterol biosynthesis by the soil amoeba Acanthamoeba polyphaga.
Eur. J. Biochem. 164(2) , 421-6, (1987) Tridemorph and fenpropimorph, two systemic fungicides known by their inhibitory effects on sterol biosynthesis in fungi and plants, were administered in vivo to the amoeba Acanthamoeba polyphaga. The compounds did not kill the cells, but modified completely t... |
|
|
An embryotoxicity study of the fungicide tridemorph and its commercial formulation Calixin.
Teratology 29(2) , 259-69, (1984) Tridemorph (N-tridecyl-2,6-dimethylmorpholine), the active ingredient of the commercially formulated fungicide Calixin, is a teratogen in rats and mice. The no-effect level for embryotoxic effects was 27.5 mg/kg for mice and 20.6 mg/kg for rats. By contrast, ... |