One-step synthesis of [16]helicene.
Kazuyuki Mori, Takashi Murase, Makoto Fujita
Index: Angew. Chem. Int. Ed. Engl. 54 , 6847-51, (2015)
Full Text: HTML
Abstract
A single-strand arylene-vinylene precursor containing four phenylene and three naphthylene units linked together with six vinylene spacers undergoes helical folding via sextuple photocyclization to give a [16]helicene core in a single step. The phenylene and naphthylene units are arranged in the precursor such that unfavorable side reactions (anthracene or benzoperylene formation) are avoided, and this is the key to the success of the one-step synthesis of [16]helicene, which is the longest [n]helicene that has been synthesized to date. © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
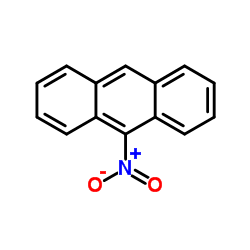 |
9-Nitroanthracene
CAS:602-60-8 |
C14H9NO2 |
|
Preparation, Structural Determination, and Characterization ...
2015-11-09 [Chemistry 21 , 16411-20, (2015)] |
|
9-Nitroanthracene derivative as a precursor of anthraquinone...
2007-06-01 [Bioorg. Med. Chem. 15(11) , 3869-73, (2007)] |
|
Xanthine oxidase-catalyzed DNA binding of dihydrodiol deriva...
1986-11-26 [Biochem. Biophys. Res. Commun. 141(1) , 245-50, (1986)] |
|
HPLC retention behavior of poly-aromatic-hydrocarbons on ami...
2004-01-01 [Chem. Pharm. Bull. 52(1) , 41-6, (2004)] |
|
Contamination is a frequent confounding factor in toxicology...
2004-01-01 [Int. J. Toxicol. 23(5) , 335-44, (2004)] |