| Structure | Name/CAS No. | Articles |
|---|---|---|
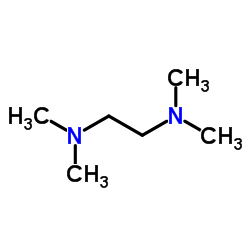 |
TMEDA
CAS:110-18-9 |
|
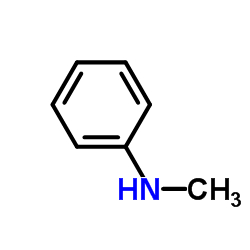 |
N-Methylaniline
CAS:100-61-8 |
|
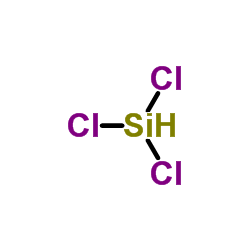 |
Trichlorosilane
CAS:10025-78-2 |