| Structure | Name/CAS No. | Articles |
|---|---|---|
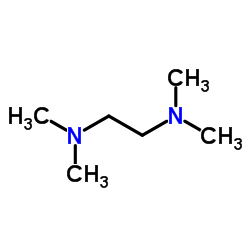 |
TMEDA
CAS:110-18-9 |
|
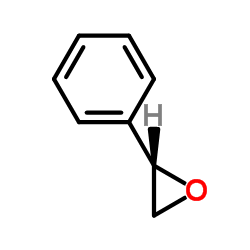 |
(S)-Styrene oxide
CAS:20780-54-5 |
|
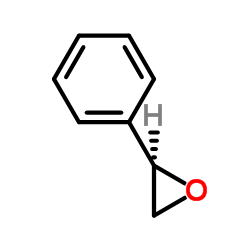 |
(S)-Styrene oxide
CAS:20780-53-4 |
|
 |
Styrene oxide
CAS:96-09-3 |