| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Potassium 3-sulfotrioxidan-1-ide
CAS:70693-62-8 |
|
 |
Pyridine hydrobromide
CAS:18820-82-1 |
|
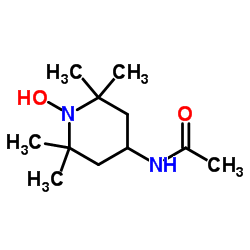 |
4-Acetamido-TEMPO, free radical
CAS:14691-89-5 |